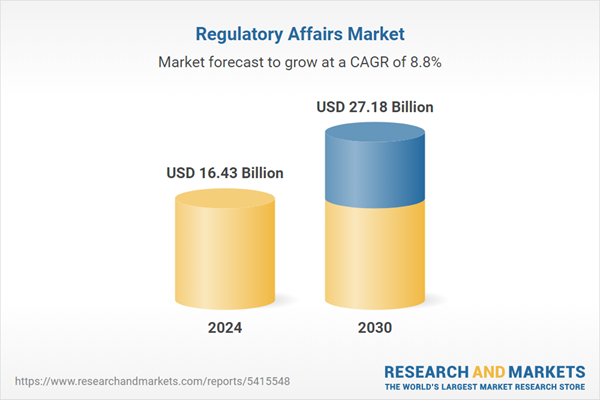
The Regulatory Affairs Market was valued at USD 16.43 Billion in 2024, and is projected to reach USD 27.18 Billion by 2030, rising at a CAGR of 8.80%. The factors expected to contribute to the growth of this market include changing regulatory requirements based on business activities and geographies, an increase in clinical trials & drug approvals along with accelerated regulatory approval, and technological advancement in regulatory software. Also, the evolution of personalized medicines, the increasing need for companies to focus on core business activities, and economic and competitive pressures are other factors that are contributing to the growth of the market.
The pharmaceutical and regulatory agencies joined forces to rapidly develop vaccines and medical products required to fight against the COVID-19 infection. The regulatory authorities take numerous precautions to ensure patient and personnel safety during a clinical trial, as well as data integrity and good laboratory practices are maintained. Growth in markets for biosimilars, orphan drugs, personalized medicines, companion diagnostics, and adaptive trial designs is projected to boost the demand for regulatory specialization in these areas. As companies venture into newer fields, the growing need to comply with regulations is boosting the demand for specialized service providers with expertise in regulatory affairs. Patent expiration of biologics, such as Simulect, Vectibix, Mircera, and Kineret, is increasing the demand and development of biosimilars, thereby contributing to the demand for regulatory services.
Several companies are actively involved in collaborations and new product development to gain leadership in the personalized medicine market, indicating a need for supportive regulatory affairs. For instance, in May 2020, Regeneron Pharmaceuticals, Inc. collaborated with Colorado Center for Personalized Medicine to design advancements in personalized medicine and human genetics.
This product will be delivered within 1-3 business days.
The pharmaceutical and regulatory agencies joined forces to rapidly develop vaccines and medical products required to fight against the COVID-19 infection. The regulatory authorities take numerous precautions to ensure patient and personnel safety during a clinical trial, as well as data integrity and good laboratory practices are maintained. Growth in markets for biosimilars, orphan drugs, personalized medicines, companion diagnostics, and adaptive trial designs is projected to boost the demand for regulatory specialization in these areas. As companies venture into newer fields, the growing need to comply with regulations is boosting the demand for specialized service providers with expertise in regulatory affairs. Patent expiration of biologics, such as Simulect, Vectibix, Mircera, and Kineret, is increasing the demand and development of biosimilars, thereby contributing to the demand for regulatory services.
Several companies are actively involved in collaborations and new product development to gain leadership in the personalized medicine market, indicating a need for supportive regulatory affairs. For instance, in May 2020, Regeneron Pharmaceuticals, Inc. collaborated with Colorado Center for Personalized Medicine to design advancements in personalized medicine and human genetics.
Regulatory Affairs Market Report Highlights
- The outsourcing segment dominated the market with a share of 59.05% in 2024. The growth of the segment is mainly due to the growing focus of the pharmaceutical and medical device companies to outsource their activities as it allows access to specialized expertise and resources that they may not have in-house.
- The regulatory writing & publishing segment held a significant share of the market in 2024. The growth of the segment is attributed to several pharmaceutical or biopharmaceutical companies reducing costs, prioritizing strategic projects, reducing staff training time, and improving overall efficiency, as well as providing greater flexibility.
- The drugs segment dominated the regulatory affairs market in 2024. The growth of the segment can be attributed to various regulations and related regulatory submissions/documentation at each of the steps involved in the process.
- The oncology segment dominated the market in 2024. The growth of the segment is due to the increasing prevalence of cancer, which requires effective and safe treatment options.
- Clinical studies dominated the market in 2024. The increasing prevalence of chronic diseases coupled with the emergence of new diseases will increase the demand for better treatment options, further growing the number of clinical trials conducted globally to meet the growing needs of the patients.
- The medium company size segment dominated the market in 2024. The growth of the market is mainly due to the strong presence of several mid-sized established firms, mainly which are privately held.
- The pharmaceutical companies segment dominated the market in 2024. The growth of the segment is due to growing research and development activities coupled with an increase in the number of approved pharmaceutical products.
- The regulatory affairs market in Asia-Pacific dominated the global industry and accounted for a 38.03% share in 2024.
Why Should You Buy This Report?
- Comprehensive Market Analysis: Gain detailed insights into the market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listings for you to stay ahead of the curve
This product will be delivered within 1-3 business days.
Table of Contents
Chapter 1. Methodology and Scope
1.1. Market Segmentation & Scope
1.1.1. Regional Scope
1.1.2. Estimates and Forecast Timeline
1.2. Market Definitions
1.3. Research Methodology
1.3.1. Information Procurement
1.3.2. Purchased Database
1.3.3. Internal Database
1.3.4. Secondary Sources
1.3.5. Primary Research
1.4. Information or Data Analysis
1.4.1. Information Analysis
1.4.2. Market Formulation & Data Visualization
1.4.3. Data Validation & Publishing
1.5. Model Details
1.5.1. Commodity Flow Analysis (Model 1)
1.5.2. Top Down Market Estimation (Model 2)
1.5.3. Value-Chain-Based Sizing & Forecasting (Model 3)
1.5.4. Multivariate Analysis (Model 4)
1.6. List of Secondary Sources
1.7. List of Abbreviations
1.8. Objectives
1.1.1. Regional Scope
1.1.2. Estimates and Forecast Timeline
1.2. Market Definitions
1.3. Research Methodology
1.3.1. Information Procurement
1.3.2. Purchased Database
1.3.3. Internal Database
1.3.4. Secondary Sources
1.3.5. Primary Research
1.4. Information or Data Analysis
1.4.1. Information Analysis
1.4.2. Market Formulation & Data Visualization
1.4.3. Data Validation & Publishing
1.5. Model Details
1.5.1. Commodity Flow Analysis (Model 1)
1.5.2. Top Down Market Estimation (Model 2)
1.5.3. Value-Chain-Based Sizing & Forecasting (Model 3)
1.5.4. Multivariate Analysis (Model 4)
1.6. List of Secondary Sources
1.7. List of Abbreviations
1.8. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Snapshot
2.3. Competitive Insights Landscape
2.2. Segment Snapshot
2.3. Competitive Insights Landscape
Chapter 3. Regulatory Affairs Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook.
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Changing regulatory landscape
3.2.1.2. Entry of companies into The global market
3.2.1.3. Life science companies focusing on their core competencies
3.2.1.4. Economic and competitive pressures
3.2.1.5. Demand for The faster approval process for breakthrough drugs and devices
3.2.1.6. Growth in emerging areas such as personalized medicine, biosimilars, and orphan drugs
3.2.2. Market Restraint Analysis
3.2.2.1. Risk Associated with Data Security
3.2.2.2. Monitoring Issues and Lack of Standardization
3.3. Industry Market Analysis Tools
3.3.1. Industry Analysis - Porter’s
3.3.2. PESTEL Analysis
3.3.3. COVID-19 Impact Analysis
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook.
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Changing regulatory landscape
3.2.1.2. Entry of companies into The global market
3.2.1.3. Life science companies focusing on their core competencies
3.2.1.4. Economic and competitive pressures
3.2.1.5. Demand for The faster approval process for breakthrough drugs and devices
3.2.1.6. Growth in emerging areas such as personalized medicine, biosimilars, and orphan drugs
3.2.2. Market Restraint Analysis
3.2.2.1. Risk Associated with Data Security
3.2.2.2. Monitoring Issues and Lack of Standardization
3.3. Industry Market Analysis Tools
3.3.1. Industry Analysis - Porter’s
3.3.2. PESTEL Analysis
3.3.3. COVID-19 Impact Analysis
Chapter 4. Regulatory Affairs Market: Services Estimates & Trend Analysis
4.1. Segment Dashboard
4.2. Global Regulatory Affairs Market Services Movement Analysis
4.3. Global Regulatory Affairs Market Size & Trend Analysis, by Services, 2018 to 2030 (USD Million)
4.4. Regulatory Consulting
4.4.1. Regulatory consulting market estimates and forecasts, 2018-2030 (USD Million)
4.5. Legal Representation
4.5.1. Legal representation market estimates and forecasts, 2018-2030 (USD Million)
4.6. Regulatory Writing & Publishing
4.6.1. Regulatory writing & publishing market estimates and forecasts, 2018-2030 (USD Million)
4.6.1.1. Writing
4.6.1.1.1. Writing market estimates and forecasts, 2018-2030 (USD Million)
4.6.1.2. Publishing
4.6.1.2.1. Publishing market estimates and forecasts, 2018-2030 (USD Million)
4.7. Product Registration & Clinical Trial Applications
4.7.1. Product registration & clinical trial applications market estimates and forecasts, 2018-2030 (USD Million)
4.8. Other Services
4.8.1. Other services market estimates and forecasts, 2018-2030 (USD Million)
4.2. Global Regulatory Affairs Market Services Movement Analysis
4.3. Global Regulatory Affairs Market Size & Trend Analysis, by Services, 2018 to 2030 (USD Million)
4.4. Regulatory Consulting
4.4.1. Regulatory consulting market estimates and forecasts, 2018-2030 (USD Million)
4.5. Legal Representation
4.5.1. Legal representation market estimates and forecasts, 2018-2030 (USD Million)
4.6. Regulatory Writing & Publishing
4.6.1. Regulatory writing & publishing market estimates and forecasts, 2018-2030 (USD Million)
4.6.1.1. Writing
4.6.1.1.1. Writing market estimates and forecasts, 2018-2030 (USD Million)
4.6.1.2. Publishing
4.6.1.2.1. Publishing market estimates and forecasts, 2018-2030 (USD Million)
4.7. Product Registration & Clinical Trial Applications
4.7.1. Product registration & clinical trial applications market estimates and forecasts, 2018-2030 (USD Million)
4.8. Other Services
4.8.1. Other services market estimates and forecasts, 2018-2030 (USD Million)
Chapter 5. Regulatory Affairs Market: Category Estimates & Trend Analysis
5.1. Segment Dashboard
5.2. Global Regulatory Affairs Market Category Movement Analysis
5.3. Global Regulatory Affairs Market Size & Trend Analysis, by Category, 2018 to 2030 (USD Million)
5.4. Drugs
5.4.1. Drugs market estimates and forecasts, 2018-2030 (USD Million)
5.4.2. Innovator
5.4.2.1. Innovator market estimates and forecasts, 2018-2030 (USD Million)
5.4.2.2. Preclinical
5.4.2.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.4.2.3. Clinical
5.4.2.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.4.2.4. Pre-Market Approval (PMA)
5.4.2.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.4.3. Generics
5.4.3.1. Generics market estimates and forecasts, 2018-2030 (USD Million)
5.4.3.2. Preclinical
5.4.3.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.4.3.3. Clinical
5.4.3.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.4.3.4. Pre-Market Approval (PMA)
5.4.3.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.5. Biologics
5.5.1. Biologics market estimates and forecasts, 2018-2030 (USD Million)
5.5.2. Biotech
5.5.2.1. Biotech market estimates and forecasts, 2018-2030 (USD Million)
5.5.2.2. Preclinical
5.5.2.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.5.2.3. Clinical
5.5.2.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.5.2.4. Pre-Market Approval (PMA)
5.5.2.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.5.3. ATMP
5.5.3.1. ATMP market estimates and forecasts, 2018-2030 (USD Million)
5.5.3.2. Preclinical
5.5.3.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.5.3.3. Clinical
5.5.3.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.5.3.4. Pre-Market Approval (PMA)
5.5.3.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.6. Biosimilars
5.6.1. Biosimilars market estimates and forecasts, 2018-2030 (USD Million)
5.6.1.1. Preclinical
5.6.1.1.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.6.1.2. Clinical
5.6.1.2.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.6.1.3. Pre-Market Approval (PMA)
5.6.1.3.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.7. Medical Devices
5.7.1. Medical devices market estimates and forecasts, 2018-2030 (USD Million)
5.7.2. Diagnostics
5.7.2.1. Diagnostics market estimates and forecasts, 2018-2030 (USD Million)
5.7.2.2. Preclinical
5.7.2.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.7.2.3. Clinical
5.7.2.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.7.2.4. Pre-Market Approval (PMA)
5.7.2.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.7.3. Therapeutics
5.7.3.1. Therapeutics market estimates and forecasts, 2018-2030 (USD Million)
5.7.3.2. Preclinical
5.7.3.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.7.3.3. Clinical
5.7.3.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.7.3.4. Pre-Market Approval (PMA)
5.7.3.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.2. Global Regulatory Affairs Market Category Movement Analysis
5.3. Global Regulatory Affairs Market Size & Trend Analysis, by Category, 2018 to 2030 (USD Million)
5.4. Drugs
5.4.1. Drugs market estimates and forecasts, 2018-2030 (USD Million)
5.4.2. Innovator
5.4.2.1. Innovator market estimates and forecasts, 2018-2030 (USD Million)
5.4.2.2. Preclinical
5.4.2.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.4.2.3. Clinical
5.4.2.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.4.2.4. Pre-Market Approval (PMA)
5.4.2.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.4.3. Generics
5.4.3.1. Generics market estimates and forecasts, 2018-2030 (USD Million)
5.4.3.2. Preclinical
5.4.3.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.4.3.3. Clinical
5.4.3.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.4.3.4. Pre-Market Approval (PMA)
5.4.3.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.5. Biologics
5.5.1. Biologics market estimates and forecasts, 2018-2030 (USD Million)
5.5.2. Biotech
5.5.2.1. Biotech market estimates and forecasts, 2018-2030 (USD Million)
5.5.2.2. Preclinical
5.5.2.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.5.2.3. Clinical
5.5.2.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.5.2.4. Pre-Market Approval (PMA)
5.5.2.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.5.3. ATMP
5.5.3.1. ATMP market estimates and forecasts, 2018-2030 (USD Million)
5.5.3.2. Preclinical
5.5.3.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.5.3.3. Clinical
5.5.3.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.5.3.4. Pre-Market Approval (PMA)
5.5.3.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.6. Biosimilars
5.6.1. Biosimilars market estimates and forecasts, 2018-2030 (USD Million)
5.6.1.1. Preclinical
5.6.1.1.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.6.1.2. Clinical
5.6.1.2.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.6.1.3. Pre-Market Approval (PMA)
5.6.1.3.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.7. Medical Devices
5.7.1. Medical devices market estimates and forecasts, 2018-2030 (USD Million)
5.7.2. Diagnostics
5.7.2.1. Diagnostics market estimates and forecasts, 2018-2030 (USD Million)
5.7.2.2. Preclinical
5.7.2.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.7.2.3. Clinical
5.7.2.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.7.2.4. Pre-Market Approval (PMA)
5.7.2.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
5.7.3. Therapeutics
5.7.3.1. Therapeutics market estimates and forecasts, 2018-2030 (USD Million)
5.7.3.2. Preclinical
5.7.3.2.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
5.7.3.3. Clinical
5.7.3.3.1. Clinical market estimates and forecasts, 2018-2030 (USD Million)
5.7.3.4. Pre-Market Approval (PMA)
5.7.3.4.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
Chapter 6. Regulatory Affairs Market: Indication Estimates & Trend Analysis
6.1. Segment Dashboard
6.2. Global Regulatory Affairs Market Indication Movement Analysis
6.3. Global Regulatory Affairs Market Size & Trend Analysis, by Indication, 2018 to 2030 (USD Million)
6.4. Oncology
6.4.1. Oncology market estimates and forecasts, 2018-2030 (USD Million)
6.5. Neurology
6.5.1. Neurology market estimates and forecasts, 2018-2030 (USD Million)
6.6. Cardiology
6.6.1. Cardiology market estimates and forecasts, 2018-2030 (USD Million)
6.7. Immunology
6.7.1. Immunology market estimates and forecasts, 2018-2030 (USD Million)
6.8. Others
6.8.1.1. Others market estimates and forecasts, 2018-2030 (USD Million)
6.2. Global Regulatory Affairs Market Indication Movement Analysis
6.3. Global Regulatory Affairs Market Size & Trend Analysis, by Indication, 2018 to 2030 (USD Million)
6.4. Oncology
6.4.1. Oncology market estimates and forecasts, 2018-2030 (USD Million)
6.5. Neurology
6.5.1. Neurology market estimates and forecasts, 2018-2030 (USD Million)
6.6. Cardiology
6.6.1. Cardiology market estimates and forecasts, 2018-2030 (USD Million)
6.7. Immunology
6.7.1. Immunology market estimates and forecasts, 2018-2030 (USD Million)
6.8. Others
6.8.1.1. Others market estimates and forecasts, 2018-2030 (USD Million)
Chapter 7. Regulatory Affairs Market: Product Stage Estimates & Trend Analysis
7.1. Segment Dashboard
7.2. Global Regulatory Affairs Market Product Stage Movement Analysis
7.3. Global Regulatory Affairs Market Size & Trend Analysis, by Product Stage, 2018 to 2030 (USD Million)
7.4. Preclinical
7.4.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
7.5. Clinical studies
7.5.1. Clinical studies market estimates and forecasts, 2018-2030 (USD Million)
7.6. Pre-Market Approval (PMA)
7.6.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
7.2. Global Regulatory Affairs Market Product Stage Movement Analysis
7.3. Global Regulatory Affairs Market Size & Trend Analysis, by Product Stage, 2018 to 2030 (USD Million)
7.4. Preclinical
7.4.1. Preclinical market estimates and forecasts, 2018-2030 (USD Million)
7.5. Clinical studies
7.5.1. Clinical studies market estimates and forecasts, 2018-2030 (USD Million)
7.6. Pre-Market Approval (PMA)
7.6.1. Pre-Market Approval (PMA) market estimates and forecasts, 2018-2030 (USD Million)
Chapter 8. Regulatory Affairs Market: Type Estimates & Trend Analysis
8.1. Segment Dashboard
8.2. Global Regulatory Affairs Market Type Movement Analysis
8.3. Global Regulatory Affairs Market Size & Trend Analysis, by Type, 2018 to 2030 (USD Million)
8.4. in-house
8.4.1. in-house market estimates and forecasts, 2018-2030 (USD Million)
8.5. Outsourced
8.5.1. Outsourced market estimates and forecasts, 2018-2030 (USD Million)
8.2. Global Regulatory Affairs Market Type Movement Analysis
8.3. Global Regulatory Affairs Market Size & Trend Analysis, by Type, 2018 to 2030 (USD Million)
8.4. in-house
8.4.1. in-house market estimates and forecasts, 2018-2030 (USD Million)
8.5. Outsourced
8.5.1. Outsourced market estimates and forecasts, 2018-2030 (USD Million)
Chapter 9. Regulatory Affairs Market: Company Size Estimates & Trend Analysis
9.1. Segment Dashboard
9.2. Global Regulatory Affairs Market Company Size Movement Analysis
9.3. Global Regulatory Affairs Market Size & Trend Analysis, by Company Size, 2018 to 2030 (USD Million)
9.4. Small
9.4.1. Small market estimates and forecasts, 2018-2030 (USD Million)
9.5. Medium
9.5.1. Medium market estimates and forecasts, 2018-2030 (USD Million)
9.6. Large
9.6.1. Large market estimates and forecasts, 2018-2030 (USD Million)
9.2. Global Regulatory Affairs Market Company Size Movement Analysis
9.3. Global Regulatory Affairs Market Size & Trend Analysis, by Company Size, 2018 to 2030 (USD Million)
9.4. Small
9.4.1. Small market estimates and forecasts, 2018-2030 (USD Million)
9.5. Medium
9.5.1. Medium market estimates and forecasts, 2018-2030 (USD Million)
9.6. Large
9.6.1. Large market estimates and forecasts, 2018-2030 (USD Million)
Chapter 10. Regulatory Affairs Market: End-use Estimates & Trend Analysis
10.1. Segment Dashboard
10.2. Global Regulatory Affairs Market End-use Movement Analysis
10.3. Global Regulatory Affairs Market Size & Trend Analysis, by End-use, 2018 to 2030 (USD Million)
10.4. Medical Device Companies
10.4.1. Medical device companies market estimates and forecasts, 2018-2030 (USD Million)
10.5. Pharmaceutical Companies
10.5.1. Pharmaceutical companies market estimates and forecasts, 2018-2030 (USD Million)
10.6. Biotechnology Companies
10.6.1. Biotechnology companies market estimates and forecasts, 2018-2030 (USD Million)
10.2. Global Regulatory Affairs Market End-use Movement Analysis
10.3. Global Regulatory Affairs Market Size & Trend Analysis, by End-use, 2018 to 2030 (USD Million)
10.4. Medical Device Companies
10.4.1. Medical device companies market estimates and forecasts, 2018-2030 (USD Million)
10.5. Pharmaceutical Companies
10.5.1. Pharmaceutical companies market estimates and forecasts, 2018-2030 (USD Million)
10.6. Biotechnology Companies
10.6.1. Biotechnology companies market estimates and forecasts, 2018-2030 (USD Million)
Chapter 11. Regulatory Affairs Market: Regional Estimates & Trend Analysis
11.1. Regional Market Share Analysis, 2024 & 2030
11.2. Regional Market Dashboard
11.3. Market Size & Forecasts Trend Analysis, 2018 to 2030 (USD Million)
11.4. North America
11.4.1. North America Market Estimates and Forecasts 2018 to 2030, (USD Million)
11.4.2. U.S.
11.4.2.1. Key country dynamics
11.4.2.2. Competitive scenario
11.4.2.3. Regulatory scenario
11.4.2.4. U.S. market estimates and forecasts, 2018-2030 (USD Million)
11.4.3. Canada
11.4.3.1. Key country dynamics
11.4.3.2. Competitive scenario
11.4.3.3. Regulatory scenario
11.4.3.4. Canada market estimates and forecasts, 2018-2030 (USD Million)
11.4.4. Mexico
11.4.4.1. Key country dynamics
11.4.4.2. Competitive scenario
11.4.4.3. Regulatory scenario
11.4.4.4. Mexico market estimates and forecasts, 2018-2030 (USD Million)
11.5. Europe
11.5.1. Europe Market Estimates and Forecasts 2018 to 2030 (USD Million)
11.5.2. UK
11.5.2.1. Key country dynamics
11.5.2.2. Competitive scenario
11.5.2.3. Regulatory scenario
11.5.2.4. UK market estimates and forecasts, 2018-2030 (USD Million)
11.5.3. Germany
11.5.3.1. Key country dynamics
11.5.3.2. Competitive scenario
11.5.3.3. Regulatory scenario
11.5.3.4. Germany market estimates and forecasts, 2018-2030 (USD Million)
11.5.4. France
11.5.4.1. Key country dynamics
11.5.4.2. Competitive scenario
11.5.4.3. Regulatory scenario
11.5.4.4. France market estimates and forecasts, 2018-2030 (USD Million)
11.5.5. Italy
11.5.5.1. Key country dynamics
11.5.5.2. Competitive scenario
11.5.5.3. Regulatory scenario
11.5.5.4. Italy market estimates and forecasts, 2018-2030 (USD Million)
11.5.6. Spain
11.5.6.1. Key country dynamics
11.5.6.2. Competitive scenario
11.5.6.3. Regulatory scenario
11.5.6.4. Spain market estimates and forecasts, 2018-2030 (USD Million)
11.5.7. Denmark
11.5.7.1. Key country dynamics
11.5.7.2. Competitive scenario
11.5.7.3. Regulatory scenario
11.5.7.4. Denmark market estimates and forecasts, 2018-2030 (USD Million)
11.5.8. Norway
11.5.8.1. Key country dynamics
11.5.8.2. Competitive scenario
11.5.8.3. Regulatory scenario
11.5.8.4. Norway market estimates and forecasts, 2018-2030 (USD Million)
11.5.9. Netherlands
11.5.9.1. Key country dynamics
11.5.9.2. Competitive scenario
11.5.9.3. Regulatory scenario
11.5.9.4. Netherlands market estimates and forecasts, 2018-2030 (USD Million)
11.5.10. Switzerland
11.5.10.1. Key country dynamics
11.5.10.2. Competitive scenario
11.5.10.3. Regulatory scenario
11.5.10.4. Switzerland market estimates and forecasts, 2018-2030 (USD Million)
11.5.11. Sweden
11.5.11.1. Key country dynamics
11.5.11.2. Competitive scenario
11.5.11.3. Regulatory scenario
11.5.11.4. Sweden market estimates and forecasts, 2018-2030 (USD Million)
11.6. Asia Pacific
11.6.1. Asia Pacific Market Estimates and Forecasts 2018 to 2030 (USD Million)
11.6.2. Japan
11.6.2.1. Key country dynamics
11.6.2.2. Competitive scenario
11.6.2.3. Regulatory scenario
11.6.2.4. Japan market estimates and forecasts, 2018-2030 (USD Million)
11.6.3. China
11.6.3.1. Key country dynamics
11.6.3.2. Competitive scenario
11.6.3.3. Regulatory scenario
11.6.3.4. China market estimates and forecasts, 2018-2030 (USD Million)
11.6.4. India
11.6.4.1. Key country dynamics
11.6.4.2. Competitive scenario
11.6.4.3. Regulatory scenario
11.6.4.4. India market estimates and forecasts, 2018-2030 (USD Million)
11.6.5. Australia
11.6.5.1. Key country dynamics
11.6.5.2. Competitive scenario
11.6.5.3. Regulatory scenario
11.6.5.4. Australia market estimates and forecasts, 2018-2030 (USD Million)
11.6.6. South Korea
11.6.6.1. Key country dynamics
11.6.6.2. Competitive scenario
11.6.6.3. Regulatory scenario
11.6.6.4. South Korea market estimates and forecasts, 2018-2030 (USD Million)
11.6.7. Thailand
11.6.7.1. Key country dynamics
11.6.7.2. Competitive scenario
11.6.7.3. Regulatory scenario
11.6.7.4. Thailand market estimates and forecasts, 2018-2030 (USD Million)
11.6.8. Indonesia
11.6.8.1. Key country dynamics
11.6.8.2. Competitive scenario
11.6.8.3. Regulatory scenario
11.6.8.4. Indonesia market estimates and forecasts, 2018-2030 (USD Million)
11.6.9. Malaysia
11.6.9.1. Key country dynamics
11.6.9.2. Competitive scenario
11.6.9.3. Regulatory scenario
11.6.9.4. Malaysia market estimates and forecasts, 2018-2030 (USD Million)
11.6.10. Singapore
11.6.10.1. Key country dynamics
11.6.10.2. Competitive scenario
11.6.10.3. Regulatory scenario
11.6.10.4. Singapore market estimates and forecasts, 2018-2030 (USD Million)
11.6.11. Taiwan
11.6.11.1. Key country dynamics
11.6.11.2. Competitive scenario
11.6.11.3. Regulatory scenario
11.6.11.4. Taiwan market estimates and forecasts, 2018-2030 (USD Million)
11.7. Latin America
11.7.1. Latin America Market Estimates and Forecasts 2018 to 2030 (USD Million)
11.7.2. Brazil
11.7.2.1. Key country dynamics
11.7.2.2. Competitive scenario
11.7.2.3. Regulatory scenario
11.7.2.4. Brazil market estimates and forecasts, 2018-2030 (USD Million)
11.7.3. Argentina
11.7.3.1. Key country dynamics
11.7.3.2. Competitive scenario
11.7.3.3. Regulatory scenario
11.7.3.4. Argentina market estimates and forecasts, 2018-2030 (USD Million)
11.7.4. Colombia
11.7.4.1. Key country dynamics
11.7.4.2. Competitive scenario
11.7.4.3. Regulatory scenario
11.7.4.4. Colombia market estimates and forecasts, 2018-2030 (USD Million)
11.7.5. Chile
11.7.5.1. Key country dynamics
11.7.5.2. Competitive scenario
11.7.5.3. Regulatory scenario
11.7.5.4. Chile market estimates and forecasts, 2018-2030 (USD Million)
11.8. MEA
11.8.1. MEA Market Estimates and Forecasts 2018 to 2030 (USD Million)
11.8.2. South Africa
11.8.2.1. Key country dynamics
11.8.2.2. Competitive scenario
11.8.2.3. Regulatory scenario
11.8.2.4. South Africa market estimates and forecasts, 2018-2030 (USD Million)
11.8.3. Saudi Arabia
11.8.3.1. Key country dynamics
11.8.3.2. Competitive scenario
11.8.3.3. Regulatory scenario
11.8.3.4. Saudi Arabia market estimates and forecasts, 2018-2030 (USD Million)
11.8.4. UAE
11.8.4.1. Key country dynamics
11.8.4.2. Competitive scenario
11.8.4.3. Regulatory scenario
11.8.4.4. UAE market estimates and forecasts, 2018-2030 (USD Million)
11.8.5. Egypt
11.8.5.1. Key country dynamics
11.8.5.2. Competitive scenario
11.8.5.3. Regulatory scenario
11.8.5.4. Egypt market estimates and forecasts, 2018-2030 (USD Million)
11.8.6. Israel
11.8.6.1. Key country dynamics
11.8.6.2. Competitive scenario
11.8.6.3. Regulatory scenario
11.8.6.4. Israel market estimates and forecasts, 2018-2030 (USD Million)
11.8.7. Kuwait
11.8.7.1. Key country dynamics
11.8.7.2. Competitive scenario
11.8.7.3. Regulatory scenario
11.8.7.4. Israel market estimates and forecasts, 2018-2030 (USD Million)
11.2. Regional Market Dashboard
11.3. Market Size & Forecasts Trend Analysis, 2018 to 2030 (USD Million)
11.4. North America
11.4.1. North America Market Estimates and Forecasts 2018 to 2030, (USD Million)
11.4.2. U.S.
11.4.2.1. Key country dynamics
11.4.2.2. Competitive scenario
11.4.2.3. Regulatory scenario
11.4.2.4. U.S. market estimates and forecasts, 2018-2030 (USD Million)
11.4.3. Canada
11.4.3.1. Key country dynamics
11.4.3.2. Competitive scenario
11.4.3.3. Regulatory scenario
11.4.3.4. Canada market estimates and forecasts, 2018-2030 (USD Million)
11.4.4. Mexico
11.4.4.1. Key country dynamics
11.4.4.2. Competitive scenario
11.4.4.3. Regulatory scenario
11.4.4.4. Mexico market estimates and forecasts, 2018-2030 (USD Million)
11.5. Europe
11.5.1. Europe Market Estimates and Forecasts 2018 to 2030 (USD Million)
11.5.2. UK
11.5.2.1. Key country dynamics
11.5.2.2. Competitive scenario
11.5.2.3. Regulatory scenario
11.5.2.4. UK market estimates and forecasts, 2018-2030 (USD Million)
11.5.3. Germany
11.5.3.1. Key country dynamics
11.5.3.2. Competitive scenario
11.5.3.3. Regulatory scenario
11.5.3.4. Germany market estimates and forecasts, 2018-2030 (USD Million)
11.5.4. France
11.5.4.1. Key country dynamics
11.5.4.2. Competitive scenario
11.5.4.3. Regulatory scenario
11.5.4.4. France market estimates and forecasts, 2018-2030 (USD Million)
11.5.5. Italy
11.5.5.1. Key country dynamics
11.5.5.2. Competitive scenario
11.5.5.3. Regulatory scenario
11.5.5.4. Italy market estimates and forecasts, 2018-2030 (USD Million)
11.5.6. Spain
11.5.6.1. Key country dynamics
11.5.6.2. Competitive scenario
11.5.6.3. Regulatory scenario
11.5.6.4. Spain market estimates and forecasts, 2018-2030 (USD Million)
11.5.7. Denmark
11.5.7.1. Key country dynamics
11.5.7.2. Competitive scenario
11.5.7.3. Regulatory scenario
11.5.7.4. Denmark market estimates and forecasts, 2018-2030 (USD Million)
11.5.8. Norway
11.5.8.1. Key country dynamics
11.5.8.2. Competitive scenario
11.5.8.3. Regulatory scenario
11.5.8.4. Norway market estimates and forecasts, 2018-2030 (USD Million)
11.5.9. Netherlands
11.5.9.1. Key country dynamics
11.5.9.2. Competitive scenario
11.5.9.3. Regulatory scenario
11.5.9.4. Netherlands market estimates and forecasts, 2018-2030 (USD Million)
11.5.10. Switzerland
11.5.10.1. Key country dynamics
11.5.10.2. Competitive scenario
11.5.10.3. Regulatory scenario
11.5.10.4. Switzerland market estimates and forecasts, 2018-2030 (USD Million)
11.5.11. Sweden
11.5.11.1. Key country dynamics
11.5.11.2. Competitive scenario
11.5.11.3. Regulatory scenario
11.5.11.4. Sweden market estimates and forecasts, 2018-2030 (USD Million)
11.6. Asia Pacific
11.6.1. Asia Pacific Market Estimates and Forecasts 2018 to 2030 (USD Million)
11.6.2. Japan
11.6.2.1. Key country dynamics
11.6.2.2. Competitive scenario
11.6.2.3. Regulatory scenario
11.6.2.4. Japan market estimates and forecasts, 2018-2030 (USD Million)
11.6.3. China
11.6.3.1. Key country dynamics
11.6.3.2. Competitive scenario
11.6.3.3. Regulatory scenario
11.6.3.4. China market estimates and forecasts, 2018-2030 (USD Million)
11.6.4. India
11.6.4.1. Key country dynamics
11.6.4.2. Competitive scenario
11.6.4.3. Regulatory scenario
11.6.4.4. India market estimates and forecasts, 2018-2030 (USD Million)
11.6.5. Australia
11.6.5.1. Key country dynamics
11.6.5.2. Competitive scenario
11.6.5.3. Regulatory scenario
11.6.5.4. Australia market estimates and forecasts, 2018-2030 (USD Million)
11.6.6. South Korea
11.6.6.1. Key country dynamics
11.6.6.2. Competitive scenario
11.6.6.3. Regulatory scenario
11.6.6.4. South Korea market estimates and forecasts, 2018-2030 (USD Million)
11.6.7. Thailand
11.6.7.1. Key country dynamics
11.6.7.2. Competitive scenario
11.6.7.3. Regulatory scenario
11.6.7.4. Thailand market estimates and forecasts, 2018-2030 (USD Million)
11.6.8. Indonesia
11.6.8.1. Key country dynamics
11.6.8.2. Competitive scenario
11.6.8.3. Regulatory scenario
11.6.8.4. Indonesia market estimates and forecasts, 2018-2030 (USD Million)
11.6.9. Malaysia
11.6.9.1. Key country dynamics
11.6.9.2. Competitive scenario
11.6.9.3. Regulatory scenario
11.6.9.4. Malaysia market estimates and forecasts, 2018-2030 (USD Million)
11.6.10. Singapore
11.6.10.1. Key country dynamics
11.6.10.2. Competitive scenario
11.6.10.3. Regulatory scenario
11.6.10.4. Singapore market estimates and forecasts, 2018-2030 (USD Million)
11.6.11. Taiwan
11.6.11.1. Key country dynamics
11.6.11.2. Competitive scenario
11.6.11.3. Regulatory scenario
11.6.11.4. Taiwan market estimates and forecasts, 2018-2030 (USD Million)
11.7. Latin America
11.7.1. Latin America Market Estimates and Forecasts 2018 to 2030 (USD Million)
11.7.2. Brazil
11.7.2.1. Key country dynamics
11.7.2.2. Competitive scenario
11.7.2.3. Regulatory scenario
11.7.2.4. Brazil market estimates and forecasts, 2018-2030 (USD Million)
11.7.3. Argentina
11.7.3.1. Key country dynamics
11.7.3.2. Competitive scenario
11.7.3.3. Regulatory scenario
11.7.3.4. Argentina market estimates and forecasts, 2018-2030 (USD Million)
11.7.4. Colombia
11.7.4.1. Key country dynamics
11.7.4.2. Competitive scenario
11.7.4.3. Regulatory scenario
11.7.4.4. Colombia market estimates and forecasts, 2018-2030 (USD Million)
11.7.5. Chile
11.7.5.1. Key country dynamics
11.7.5.2. Competitive scenario
11.7.5.3. Regulatory scenario
11.7.5.4. Chile market estimates and forecasts, 2018-2030 (USD Million)
11.8. MEA
11.8.1. MEA Market Estimates and Forecasts 2018 to 2030 (USD Million)
11.8.2. South Africa
11.8.2.1. Key country dynamics
11.8.2.2. Competitive scenario
11.8.2.3. Regulatory scenario
11.8.2.4. South Africa market estimates and forecasts, 2018-2030 (USD Million)
11.8.3. Saudi Arabia
11.8.3.1. Key country dynamics
11.8.3.2. Competitive scenario
11.8.3.3. Regulatory scenario
11.8.3.4. Saudi Arabia market estimates and forecasts, 2018-2030 (USD Million)
11.8.4. UAE
11.8.4.1. Key country dynamics
11.8.4.2. Competitive scenario
11.8.4.3. Regulatory scenario
11.8.4.4. UAE market estimates and forecasts, 2018-2030 (USD Million)
11.8.5. Egypt
11.8.5.1. Key country dynamics
11.8.5.2. Competitive scenario
11.8.5.3. Regulatory scenario
11.8.5.4. Egypt market estimates and forecasts, 2018-2030 (USD Million)
11.8.6. Israel
11.8.6.1. Key country dynamics
11.8.6.2. Competitive scenario
11.8.6.3. Regulatory scenario
11.8.6.4. Israel market estimates and forecasts, 2018-2030 (USD Million)
11.8.7. Kuwait
11.8.7.1. Key country dynamics
11.8.7.2. Competitive scenario
11.8.7.3. Regulatory scenario
11.8.7.4. Israel market estimates and forecasts, 2018-2030 (USD Million)
Chapter 12. Competitive Landscape
12.1. Competition Categorization
12.1.1. Market Leaders
12.1.2. Emerging Players
12.2. Company Market Share/Assessment Analysis, 2024
12.3. Company Profiles
12.3.1. Accell Clinical Research, LLC
12.3.1.1. Company overview
12.3.1.2. Financial performance
12.3.1.3. Service benchmarking
12.3.1.4. Strategic initiatives
12.3.2. Genpact
12.3.2.1. Company overview
12.3.2.2. Financial performance
12.3.2.3. Service benchmarking
12.3.2.4. Strategic initiatives
12.3.3. Criterium, Inc.
12.3.3.1. Company overview
12.3.3.2. Financial performance
12.3.3.3. Service benchmarking
12.3.3.4. Strategic initiatives
12.3.4. ICON plc
12.3.4.1. Company overview
12.3.4.2. Financial performance
12.3.4.3. Service benchmarking
12.3.4.4. Strategic initiatives
12.3.5. iuvo BioScience, LLC.
12.3.5.1. Company overview
12.3.5.2. Financial performance
12.3.5.3. Service benchmarking
12.3.5.4. Strategic initiatives
12.3.6. WuXi AppTec
12.3.6.1. Company overview
12.3.6.2. Financial performance
12.3.6.3. Service benchmarking
12.3.6.4. Strategic initiatives
12.3.7. Medpace
12.3.7.1. Company overview
12.3.7.2. Financial performance
12.3.7.3. Service benchmarking
12.3.7.4. Strategic initiatives
12.3.8. Charles River Laboratories
12.3.8.1. Company overview
12.3.8.2. Financial performance
12.3.8.3. Service benchmarking
12.3.8.4. Strategic initiatives
12.3.9. Laboratory Corporation of America Holdings
12.3.9.1. Company overview
12.3.9.2. Financial performance
12.3.9.3. Service benchmarking
12.3.9.4. Strategic initiatives
12.3.10. Parexel International (MA) Corporation
12.3.10.1. Company overview
12.3.10.2. Financial performance
12.3.10.3. Service benchmarking
12.3.10.4. Strategic initiatives
12.3.11. Freyr
12.3.11.1. Company overview
12.3.11.2. Financial performance
12.3.11.3. Service benchmarking
12.3.11.4. Strategic initiatives
12.3.12. PHARMALEX GMBH
12.3.12.1. Company overview
12.3.12.2. Financial performance
12.3.12.3. Service benchmarking
12.3.12.4. Strategic initiatives
12.3.13. SSI Strategy LLC
12.3.13.1. Company overview
12.3.13.2. Financial performance
12.3.13.3. Service benchmarking
12.3.13.4. Strategic initiatives
12.3.14. Pharmexon
12.3.14.1. Company overview
12.3.14.2. Financial performance
12.3.14.3. Service benchmarking
12.3.14.4. Strategic initiatives
12.3.15. Qvigilance
12.3.15.1. Company overview
12.3.15.2. Financial performance
12.3.15.3. Service benchmarking
12.3.15.4. Strategic initiatives
12.3.16. BlueReg
12.3.16.1. Company overview
12.3.16.2. Financial performance
12.3.16.3. Service benchmarking
12.3.16.4. Strategic initiatives
12.3.17. Cambridge Regulatory Services
12.3.17.1. Company overview
12.3.17.2. Financial performance
12.3.17.3. Service benchmarking
12.3.17.4. Strategic initiatives
12.3.18. VCLS
12.3.18.1. Company overview
12.3.18.2. Financial performance
12.3.18.3. Service benchmarking
12.3.18.4. Strategic initiatives
12.1.1. Market Leaders
12.1.2. Emerging Players
12.2. Company Market Share/Assessment Analysis, 2024
12.3. Company Profiles
12.3.1. Accell Clinical Research, LLC
12.3.1.1. Company overview
12.3.1.2. Financial performance
12.3.1.3. Service benchmarking
12.3.1.4. Strategic initiatives
12.3.2. Genpact
12.3.2.1. Company overview
12.3.2.2. Financial performance
12.3.2.3. Service benchmarking
12.3.2.4. Strategic initiatives
12.3.3. Criterium, Inc.
12.3.3.1. Company overview
12.3.3.2. Financial performance
12.3.3.3. Service benchmarking
12.3.3.4. Strategic initiatives
12.3.4. ICON plc
12.3.4.1. Company overview
12.3.4.2. Financial performance
12.3.4.3. Service benchmarking
12.3.4.4. Strategic initiatives
12.3.5. iuvo BioScience, LLC.
12.3.5.1. Company overview
12.3.5.2. Financial performance
12.3.5.3. Service benchmarking
12.3.5.4. Strategic initiatives
12.3.6. WuXi AppTec
12.3.6.1. Company overview
12.3.6.2. Financial performance
12.3.6.3. Service benchmarking
12.3.6.4. Strategic initiatives
12.3.7. Medpace
12.3.7.1. Company overview
12.3.7.2. Financial performance
12.3.7.3. Service benchmarking
12.3.7.4. Strategic initiatives
12.3.8. Charles River Laboratories
12.3.8.1. Company overview
12.3.8.2. Financial performance
12.3.8.3. Service benchmarking
12.3.8.4. Strategic initiatives
12.3.9. Laboratory Corporation of America Holdings
12.3.9.1. Company overview
12.3.9.2. Financial performance
12.3.9.3. Service benchmarking
12.3.9.4. Strategic initiatives
12.3.10. Parexel International (MA) Corporation
12.3.10.1. Company overview
12.3.10.2. Financial performance
12.3.10.3. Service benchmarking
12.3.10.4. Strategic initiatives
12.3.11. Freyr
12.3.11.1. Company overview
12.3.11.2. Financial performance
12.3.11.3. Service benchmarking
12.3.11.4. Strategic initiatives
12.3.12. PHARMALEX GMBH
12.3.12.1. Company overview
12.3.12.2. Financial performance
12.3.12.3. Service benchmarking
12.3.12.4. Strategic initiatives
12.3.13. SSI Strategy LLC
12.3.13.1. Company overview
12.3.13.2. Financial performance
12.3.13.3. Service benchmarking
12.3.13.4. Strategic initiatives
12.3.14. Pharmexon
12.3.14.1. Company overview
12.3.14.2. Financial performance
12.3.14.3. Service benchmarking
12.3.14.4. Strategic initiatives
12.3.15. Qvigilance
12.3.15.1. Company overview
12.3.15.2. Financial performance
12.3.15.3. Service benchmarking
12.3.15.4. Strategic initiatives
12.3.16. BlueReg
12.3.16.1. Company overview
12.3.16.2. Financial performance
12.3.16.3. Service benchmarking
12.3.16.4. Strategic initiatives
12.3.17. Cambridge Regulatory Services
12.3.17.1. Company overview
12.3.17.2. Financial performance
12.3.17.3. Service benchmarking
12.3.17.4. Strategic initiatives
12.3.18. VCLS
12.3.18.1. Company overview
12.3.18.2. Financial performance
12.3.18.3. Service benchmarking
12.3.18.4. Strategic initiatives
List of Tables
Table 1 List of Secondary Sources
Table 2 List of Abbreviations
Table 3 Global Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 4 Global Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 5 Global Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 6 Global Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 7 Global Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 8 Global Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 9 Global Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 10 Global Regulatory Affairs Market, by Region, 2018-2030 (USD Million)
Table 11 North America Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 12 North America Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 13 North America Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 14 North America Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 15 North America Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 16 North America Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 17 North America Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 18 U.S. Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 19 U.S. Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 20 U.S. Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 21 U.S. Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 22 U.S. Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 23 U.S. Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 24 U.S. Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 25 Canada Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 26 Canada Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 27 Canada Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 28 Canada Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 29 Canada Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 30 Canada Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 31 Canada Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 32 Mexico Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 33 Mexico Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 34 Mexico Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 35 Mexico Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 36 Mexico Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 37 Mexico Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 38 Mexico Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 39 Europe Regulatory Affairs Market, by Country, 2018-2030 (USD Million)
Table 40 Europe Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 41 Europe Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 42 Europe Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 43 Europe Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 44 Europe Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 45 Europe Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 46 Europe Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 47 Germany Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 48 Germany Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 49 Germany Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 50 Germany Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 51 Germany Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 52 Germany Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 53 Germany Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 54 UK Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 55 UK Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 56 UK Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 57 UK Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 58 UK Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 59 UK Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 60 UK Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 61 France Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 62 France Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 63 France Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 64 France Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 65 France Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 66 France Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 67 France Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 68 Italy Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 69 Italy Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 70 Italy Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 71 Italy Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 72 Italy Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 73 Italy Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 74 Italy Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 75 Spain Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 76 Spain Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 77 Spain Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 78 Spain Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 79 Spain Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 80 Spain Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 81 Spain Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 82 Denmark Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 83 Denmark Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 84 Denmark Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 85 Denmark Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 86 Denmark Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 87 Denmark Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 88 Denmark Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 89 Norway Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 90 Norway Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 91 Norway Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 92 Norway Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 93 Norway Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 94 Norway Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 95 Norway Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 96 Netherlands Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 97 Netherlands Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 98 Netherlands Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 99 Netherlands Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 100 Netherlands Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 101 Netherlands Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 102 Netherlands Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 103 Switzerland Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 104 Switzerland Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 105 Switzerland Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 106 Switzerland Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 107 Switzerland Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 108 Switzerland Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 109 Switzerland Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 110 Sweden Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 111 Sweden Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 112 Sweden Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 113 Sweden Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 114 Sweden Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 115 Sweden Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 116 Sweden Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 117 Asia Pacific Regulatory Affairs Market, by Country, 2018-2030 (USD Million)
Table 118 Asia Pacific Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 119 Asia Pacific Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 120 Asia Pacific Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 121 Asia Pacific Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 122 Asia Pacific Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 123 Asia Pacific Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 124 Asia Pacific Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 125 China Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 126 China Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 127 China Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 128 China Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 129 China Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 130 China Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 131 China Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 132 Japan Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 133 Japan Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 134 Japan Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 135 Japan Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 136 Japan Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 137 Japan Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 138 Japan Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 139 India Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 140 India Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 141 India Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 142 India Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 143 India Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 144 India Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 145 India Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 146 South Korea Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 147 South Korea Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 148 South Korea Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 149 South Korea Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 150 South Korea Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 151 South Korea Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 152 South Korea Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 153 Australia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 154 Australia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 155 Australia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 156 Australia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 157 Australia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 158 Australia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 159 Australia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 160 Thailand Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 161 Thailand Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 162 Thailand Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 163 Thailand Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 164 Thailand Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 165 Thailand Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 166 Thailand Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 167 Indonesia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 168 Indonesia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 169 Indonesia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 170 Indonesia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 171 Indonesia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 172 Indonesia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 173 Indonesia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 174 Malaysia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 175 Malaysia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 176 Malaysia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 177 Malaysia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 178 Malaysia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 179 Malaysia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 180 Malaysia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 181 Singapore Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 182 Singapore Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 183 Singapore Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 184 Singapore Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 185 Singapore Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 186 Singapore Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 187 Singapore Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 188 Taiwan Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 189 Taiwan Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 190 Taiwan Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 191 Taiwan Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 192 Taiwan Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 193 Taiwan Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 194 Taiwan Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 195 Latin America Regulatory Affairs Market, by Country, 2018-2030 (USD Million)
Table 196 Latin America Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 197 Latin America Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 198 Latin America Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 199 Latin America Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 200 Latin America Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 201 Latin America Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 202 Latin America Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 203 Brazil Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 204 Brazil Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 205 Brazil Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 206 Brazil Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 207 Brazil Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 208 Brazil Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 209 Brazil Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 210 Argentina Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 211 Argentina Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 212 Argentina Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 213 Argentina Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 214 Argentina Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 215 Argentina Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 216 Argentina Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 217 Colombia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 218 Colombia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 219 Colombia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 220 Colombia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 221 Colombia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 222 Colombia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 223 Colombia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 224 Chile Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 225 Chile Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 226 Chile Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 227 Chile Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 228 Chile Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 229 Chile Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 230 Chile Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 231 Middle East & Africa Regulatory Affairs Market, by Country, 2018-2030 (USD Million)
Table 232 Middle East & Africa Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 233 Middle East & Africa Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 234 Middle East & Africa Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 235 Middle East & Africa Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 236 Middle East & Africa Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 237 Middle East & Africa Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 238 Middle East & Africa Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 239 South Africa Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 240 South Africa Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 241 South Africa Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 242 South Africa Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 243 South Africa Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 244 South Africa Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 245 South Africa Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 246 Saudi Arabia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 247 Saudi Arabia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 248 Saudi Arabia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 249 Saudi Arabia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 250 Saudi Arabia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 251 Saudi Arabia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 252 Saudi Arabia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 253 UAE Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 254 UAE Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 255 UAE Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 256 UAE Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 257 UAE Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 258 UAE Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 259 UAE Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 260 Egypt Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 261 Egypt Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 262 Egypt Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 263 Egypt Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 264 Egypt Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 265 Egypt Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 266 Egypt Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 267 Israel Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 268 Israel Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 269 Israel Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 270 Israel Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 271 Israel Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 272 Israel Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 273 Israel Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 274 Kuwait Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 275 Kuwait Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 276 Kuwait Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 277 Kuwait Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 278 Kuwait Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 279 Kuwait Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 280 Kuwait Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 2 List of Abbreviations
Table 3 Global Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 4 Global Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 5 Global Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 6 Global Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 7 Global Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 8 Global Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 9 Global Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 10 Global Regulatory Affairs Market, by Region, 2018-2030 (USD Million)
Table 11 North America Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 12 North America Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 13 North America Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 14 North America Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 15 North America Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 16 North America Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 17 North America Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 18 U.S. Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 19 U.S. Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 20 U.S. Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 21 U.S. Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 22 U.S. Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 23 U.S. Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 24 U.S. Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 25 Canada Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 26 Canada Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 27 Canada Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 28 Canada Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 29 Canada Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 30 Canada Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 31 Canada Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 32 Mexico Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 33 Mexico Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 34 Mexico Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 35 Mexico Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 36 Mexico Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 37 Mexico Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 38 Mexico Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 39 Europe Regulatory Affairs Market, by Country, 2018-2030 (USD Million)
Table 40 Europe Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 41 Europe Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 42 Europe Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 43 Europe Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 44 Europe Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 45 Europe Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 46 Europe Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 47 Germany Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 48 Germany Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 49 Germany Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 50 Germany Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 51 Germany Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 52 Germany Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 53 Germany Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 54 UK Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 55 UK Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 56 UK Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 57 UK Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 58 UK Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 59 UK Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 60 UK Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 61 France Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 62 France Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 63 France Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 64 France Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 65 France Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 66 France Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 67 France Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 68 Italy Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 69 Italy Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 70 Italy Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 71 Italy Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 72 Italy Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 73 Italy Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 74 Italy Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 75 Spain Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 76 Spain Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 77 Spain Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 78 Spain Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 79 Spain Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 80 Spain Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 81 Spain Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 82 Denmark Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 83 Denmark Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 84 Denmark Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 85 Denmark Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 86 Denmark Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 87 Denmark Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 88 Denmark Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 89 Norway Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 90 Norway Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 91 Norway Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 92 Norway Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 93 Norway Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 94 Norway Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 95 Norway Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 96 Netherlands Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 97 Netherlands Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 98 Netherlands Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 99 Netherlands Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 100 Netherlands Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 101 Netherlands Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 102 Netherlands Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 103 Switzerland Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 104 Switzerland Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 105 Switzerland Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 106 Switzerland Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 107 Switzerland Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 108 Switzerland Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 109 Switzerland Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 110 Sweden Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 111 Sweden Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 112 Sweden Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 113 Sweden Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 114 Sweden Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 115 Sweden Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 116 Sweden Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 117 Asia Pacific Regulatory Affairs Market, by Country, 2018-2030 (USD Million)
Table 118 Asia Pacific Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 119 Asia Pacific Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 120 Asia Pacific Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 121 Asia Pacific Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 122 Asia Pacific Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 123 Asia Pacific Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 124 Asia Pacific Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 125 China Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 126 China Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 127 China Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 128 China Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 129 China Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 130 China Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 131 China Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 132 Japan Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 133 Japan Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 134 Japan Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 135 Japan Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 136 Japan Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 137 Japan Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 138 Japan Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 139 India Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 140 India Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 141 India Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 142 India Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 143 India Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 144 India Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 145 India Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 146 South Korea Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 147 South Korea Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 148 South Korea Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 149 South Korea Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 150 South Korea Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 151 South Korea Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 152 South Korea Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 153 Australia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 154 Australia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 155 Australia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 156 Australia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 157 Australia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 158 Australia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 159 Australia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 160 Thailand Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 161 Thailand Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 162 Thailand Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 163 Thailand Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 164 Thailand Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 165 Thailand Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 166 Thailand Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 167 Indonesia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 168 Indonesia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 169 Indonesia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 170 Indonesia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 171 Indonesia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 172 Indonesia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 173 Indonesia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 174 Malaysia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 175 Malaysia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 176 Malaysia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 177 Malaysia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 178 Malaysia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 179 Malaysia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 180 Malaysia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 181 Singapore Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 182 Singapore Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 183 Singapore Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 184 Singapore Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 185 Singapore Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 186 Singapore Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 187 Singapore Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 188 Taiwan Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 189 Taiwan Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 190 Taiwan Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 191 Taiwan Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 192 Taiwan Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 193 Taiwan Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 194 Taiwan Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 195 Latin America Regulatory Affairs Market, by Country, 2018-2030 (USD Million)
Table 196 Latin America Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 197 Latin America Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 198 Latin America Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 199 Latin America Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 200 Latin America Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 201 Latin America Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 202 Latin America Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 203 Brazil Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 204 Brazil Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 205 Brazil Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 206 Brazil Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 207 Brazil Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 208 Brazil Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 209 Brazil Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 210 Argentina Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 211 Argentina Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 212 Argentina Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 213 Argentina Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 214 Argentina Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 215 Argentina Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 216 Argentina Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 217 Colombia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 218 Colombia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 219 Colombia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 220 Colombia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 221 Colombia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 222 Colombia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 223 Colombia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 224 Chile Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 225 Chile Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 226 Chile Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 227 Chile Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 228 Chile Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 229 Chile Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 230 Chile Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 231 Middle East & Africa Regulatory Affairs Market, by Country, 2018-2030 (USD Million)
Table 232 Middle East & Africa Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 233 Middle East & Africa Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 234 Middle East & Africa Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 235 Middle East & Africa Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 236 Middle East & Africa Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 237 Middle East & Africa Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 238 Middle East & Africa Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 239 South Africa Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 240 South Africa Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 241 South Africa Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 242 South Africa Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 243 South Africa Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 244 South Africa Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 245 South Africa Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 246 Saudi Arabia Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 247 Saudi Arabia Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 248 Saudi Arabia Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 249 Saudi Arabia Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 250 Saudi Arabia Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 251 Saudi Arabia Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 252 Saudi Arabia Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 253 UAE Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 254 UAE Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 255 UAE Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 256 UAE Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 257 UAE Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 258 UAE Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 259 UAE Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 260 Egypt Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 261 Egypt Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 262 Egypt Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 263 Egypt Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 264 Egypt Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 265 Egypt Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 266 Egypt Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 267 Israel Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 268 Israel Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 269 Israel Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 270 Israel Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 271 Israel Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 272 Israel Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 273 Israel Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
Table 274 Kuwait Regulatory Affairs Market, by Services, 2018-2030 (USD Million)
Table 275 Kuwait Regulatory Affairs Market, by Category, 2018-2030 (USD Million)
Table 276 Kuwait Regulatory Affairs Market, by Indication, 2018-2030 (USD Million)
Table 277 Kuwait Regulatory Affairs Market, by Product Stage, 2018-2030 (USD Million)
Table 278 Kuwait Regulatory Affairs Market, by Type, 2018-2030 (USD Million)
Table 279 Kuwait Regulatory Affairs Market, by Company Size, 2018-2030 (USD Million)
Table 280 Kuwait Regulatory Affairs Market, by End-Use, 2018-2030 (USD Million)
List of Figures
Figure 1 Regulatory Affairs Market segmentation
Figure 2 Data analysis models
Figure 3 Market formulation and validation
Figure 4 Data validating & publishing
Figure 5 Market research process
Figure 6 Information procurement
Figure 7 Primary research
Figure 8 Value-chain-based sizing & forecasting
Figure 9 QFD modelling for market share assessment
Figure 10 Market formulation & validation
Figure 11 Commodity flow analysis
Figure 12 Market outlook
Figure 13 Segment snapshot 1
Figure 14 Segment snapshot 2
Figure 15 Competitive landscape snapshot
Figure 16 Market trends & outlook
Figure 17 Porter’s five force analysis
Figure 18 PESTEL analysis
Figure 19 Regulatory Affairs Market: Services outlook key takeaways
Figure 20 Regulatory Affairs Market: Services movement analysis
Figure 21 Regulatory consulting market estimates and forecasts, 2018-2030 (USD Million)
Figure 22 Legal representation market estimates and forecasts, 2018-2030 (USD Million)
Figure 23 Regulatory writing & publishing market estimates and forecasts, 2018-2030 (USD Million)
Figure 24 Writing market estimates and forecasts, 2018-2030 (USD Million)
Figure 25 Publishing market estimates and forecasts, 2018-2030 (USD Million)
Figure 26 Product registration & clinical trial applications market estimates and forecasts, 2018-2030 (USD Million)
Figure 27 Other services market estimates and forecasts, 2018-2030 (USD Million)
Figure 28 Regulatory Affairs Market: Category outlook key takeaways
Figure 29 Regulatory Affairs Market: Category movement analysis
Figure 30 Drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 31 Innovator drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 32 Preclinical innovator drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 33 Clinical innovator drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 34 Pre-Market Approval (PMA) innovator drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 35 Generic drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 36 Preclinical generic drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 37 Clinical generic drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 38 Pre-Market Approval (PMA) generic drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 39 Biologics market estimates and forecasts, 2018-2030 (USD Million)
Figure 40 Biotech market estimates and forecasts, 2018-2030 (USD Million)
Figure 41 Preclinical biotech market estimates and forecasts, 2018-2030 (USD Million)
Figure 42 Clinical biotech market estimates and forecasts, 2018-2030 (USD Million)
Figure 43 Pre-Market Approval (PMA) biotech market estimates and forecasts, 2018-2030 (USD Million)
Figure 44 ATMP market estimates and forecasts, 2018-2030 (USD Million)
Figure 45 Preclinical ATMP market estimates and forecasts, 2018-2030 (USD Million)
Figure 46 Clinical ATMP market estimates and forecasts, 2018-2030 (USD Million)
Figure 47 Pre-Market Approval (PMA) ATMP market estimates and forecasts, 2018-2030 (USD Million)
Figure 48 Biosimilars market estimates and forecasts, 2018-2030 (USD Million)
Figure 49 Preclinical biosimilars market estimates and forecasts, 2018-2030 (USD Million)
Figure 50 Clinical biosimilars market estimates and forecasts, 2018-2030 (USD Million)
Figure 51 Pre-Market Approval (PMA) biosimilars market estimates and forecasts, 2018-2030 (USD Million)
Figure 52 Medical Devices market estimates and forecasts, 2018-2030 (USD Million)
Figure 53 Diagnostics market estimates and forecasts, 2018-2030 (USD Million)
Figure 54 Preclinical diagnostics market estimates and forecasts, 2018-2030 (USD Million)
Figure 55 Clinical diagnostics market estimates and forecasts, 2018-2030 (USD Million)
Figure 56 Pre-Market Approval (PMA) diagnostics market estimates and forecasts, 2018-2030 (USD Million)
Figure 57 Therapeutics market estimates and forecasts, 2018-2030 (USD Million)
Figure 58 Preclinical therapeutics market estimates and forecasts, 2018-2030 (USD Million)
Figure 59 Clinical therapeutics market estimates and forecasts, 2018-2030 (USD Million)
Figure 60 Pre-Market Approval (PMA) therapeutics market estimates and forecasts, 2018-2030 (USD Million)
Figure 61 Regulatory Affairs Market: Indication outlook key takeaways
Figure 62 Regulatory Affairs Market: Indication movement analysis
Figure 63 Oncology market estimates and forecasts, 2018-2030 (USD Million)
Figure 64 Neurology market estimates and forecasts, 2018-2030 (USD Million)
Figure 65 Cardiology companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 66 Immunology market estimates and forecasts, 2018-2030 (USD Million)
Figure 67 Others market estimates and forecasts, 2018-2030 (USD Million)
Figure 68 Regulatory Affairs Market: Product stage outlook key takeaways
Figure 69 Regulatory Affairs Market: Product stage movement analysis
Figure 70 Preclinical market estimates and forecasts, 2018-2030 (USD Million)
Figure 71 Clinical studies market estimates and forecasts, 2018-2030 (USD Million)
Figure 72 Pre-Market Approval (PMA) companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 73 Immunology market estimates and forecasts, 2018-2030 (USD Million)
Figure 74 Regulatory Affairs Market: Type outlook key takeaways
Figure 75 Regulatory Affairs Market: Type movement analysis
Figure 76 In-house market estimates and forecasts, 2018-2030 (USD Million)
Figure 77 Outsourced market estimates and forecasts, 2018-2030 (USD Million)
Figure 78 Regulatory Affairs Market: Company size outlook key takeaways
Figure 79 Regulatory Affairs Market: Company size movement analysis
Figure 80 Small market estimates and forecasts, 2018-2030 (USD Million)
Figure 81 Medium market estimates and forecasts, 2018-2030 (USD Million)
Figure 82 Large market estimates and forecasts, 2018-2030 (USD Million)
Figure 83 Regulatory Affairs Market: End-use outlook key takeaways
Figure 84 Regulatory Affairs Market: End-use movement analysis
Figure 85 Medical device companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 86 Pharmaceutical companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 87 Biotechnology companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 88 Regional marketplace outlook, 2024 & 2030 (USD Million)
Figure 89 Regional marketplace: Key takeaways
Figure 90 North America Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 91 Key country dynamics
Figure 92 U.S. Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 93 Key country dynamics
Figure 94 Canada Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 95 Key country dynamics
Figure 96 Mexico Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 97 Key country dynamics
Figure 98 Europe Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 99 Key country dynamics
Figure 100 UK regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 101 Key country dynamics
Figure 102 Germany Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 103 Key country dynamics
Figure 104 France Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 105 Key country dynamics
Figure 106 Italy Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 107 Key country dynamics
Figure 108 Spain Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 109 Key country dynamics
Figure 110 Denmark Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 111 Key country dynamics
Figure 112 Norway Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 113 Key country dynamics
Figure 114 Netherlands Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 115 Key country dynamics
Figure 116 Switzerland Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 117 Key country dynamics
Figure 118 Sweden Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 119 Asia Pacific Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 120 Key country dynamics
Figure 121 Japan Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 122 Key country dynamics
Figure 123 China Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 124 Key country dynamics
Figure 125 India Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 126 Key country dynamics
Figure 127 Australia Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 128 Key country dynamics
Figure 129 South Korea Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 130 Key country dynamics
Figure 131 Thailand Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 132 Key country dynamics
Figure 133 Indonesia Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 134 Key country dynamics
Figure 135 Taiwan Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 136 Key country dynamics
Figure 137 Malaysia Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 138 Key country dynamics
Figure 139 Singapore Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 140 Latin America Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 141 Key country dynamics
Figure 142 Brazil Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 143 Key country dynamics
Figure 144 Argentina Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 145 Key country dynamics
Figure 146 Colombia regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 147 Key country dynamics
Figure 148 Chile regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 149 Key country dynamics
Figure 150 MEA Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 151 Key country dynamics
Figure 152 South Africa Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 153 Key country dynamics
Figure 154 Saudi Arabia Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 155 Key country dynamics
Figure 156 UAE Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 157 Key country dynamics
Figure 158 Egypt regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 159 Key country dynamics
Figure 160 Israel regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 161 Key country dynamics
Figure 162 Kuwait regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 163 Market participant categorization
Figure 164 Regulatory affairs market share/assessment analysis, 2024
Figure 165 Strategic framework
Figure 2 Data analysis models
Figure 3 Market formulation and validation
Figure 4 Data validating & publishing
Figure 5 Market research process
Figure 6 Information procurement
Figure 7 Primary research
Figure 8 Value-chain-based sizing & forecasting
Figure 9 QFD modelling for market share assessment
Figure 10 Market formulation & validation
Figure 11 Commodity flow analysis
Figure 12 Market outlook
Figure 13 Segment snapshot 1
Figure 14 Segment snapshot 2
Figure 15 Competitive landscape snapshot
Figure 16 Market trends & outlook
Figure 17 Porter’s five force analysis
Figure 18 PESTEL analysis
Figure 19 Regulatory Affairs Market: Services outlook key takeaways
Figure 20 Regulatory Affairs Market: Services movement analysis
Figure 21 Regulatory consulting market estimates and forecasts, 2018-2030 (USD Million)
Figure 22 Legal representation market estimates and forecasts, 2018-2030 (USD Million)
Figure 23 Regulatory writing & publishing market estimates and forecasts, 2018-2030 (USD Million)
Figure 24 Writing market estimates and forecasts, 2018-2030 (USD Million)
Figure 25 Publishing market estimates and forecasts, 2018-2030 (USD Million)
Figure 26 Product registration & clinical trial applications market estimates and forecasts, 2018-2030 (USD Million)
Figure 27 Other services market estimates and forecasts, 2018-2030 (USD Million)
Figure 28 Regulatory Affairs Market: Category outlook key takeaways
Figure 29 Regulatory Affairs Market: Category movement analysis
Figure 30 Drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 31 Innovator drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 32 Preclinical innovator drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 33 Clinical innovator drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 34 Pre-Market Approval (PMA) innovator drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 35 Generic drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 36 Preclinical generic drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 37 Clinical generic drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 38 Pre-Market Approval (PMA) generic drugs market estimates and forecasts, 2018-2030 (USD Million)
Figure 39 Biologics market estimates and forecasts, 2018-2030 (USD Million)
Figure 40 Biotech market estimates and forecasts, 2018-2030 (USD Million)
Figure 41 Preclinical biotech market estimates and forecasts, 2018-2030 (USD Million)
Figure 42 Clinical biotech market estimates and forecasts, 2018-2030 (USD Million)
Figure 43 Pre-Market Approval (PMA) biotech market estimates and forecasts, 2018-2030 (USD Million)
Figure 44 ATMP market estimates and forecasts, 2018-2030 (USD Million)
Figure 45 Preclinical ATMP market estimates and forecasts, 2018-2030 (USD Million)
Figure 46 Clinical ATMP market estimates and forecasts, 2018-2030 (USD Million)
Figure 47 Pre-Market Approval (PMA) ATMP market estimates and forecasts, 2018-2030 (USD Million)
Figure 48 Biosimilars market estimates and forecasts, 2018-2030 (USD Million)
Figure 49 Preclinical biosimilars market estimates and forecasts, 2018-2030 (USD Million)
Figure 50 Clinical biosimilars market estimates and forecasts, 2018-2030 (USD Million)
Figure 51 Pre-Market Approval (PMA) biosimilars market estimates and forecasts, 2018-2030 (USD Million)
Figure 52 Medical Devices market estimates and forecasts, 2018-2030 (USD Million)
Figure 53 Diagnostics market estimates and forecasts, 2018-2030 (USD Million)
Figure 54 Preclinical diagnostics market estimates and forecasts, 2018-2030 (USD Million)
Figure 55 Clinical diagnostics market estimates and forecasts, 2018-2030 (USD Million)
Figure 56 Pre-Market Approval (PMA) diagnostics market estimates and forecasts, 2018-2030 (USD Million)
Figure 57 Therapeutics market estimates and forecasts, 2018-2030 (USD Million)
Figure 58 Preclinical therapeutics market estimates and forecasts, 2018-2030 (USD Million)
Figure 59 Clinical therapeutics market estimates and forecasts, 2018-2030 (USD Million)
Figure 60 Pre-Market Approval (PMA) therapeutics market estimates and forecasts, 2018-2030 (USD Million)
Figure 61 Regulatory Affairs Market: Indication outlook key takeaways
Figure 62 Regulatory Affairs Market: Indication movement analysis
Figure 63 Oncology market estimates and forecasts, 2018-2030 (USD Million)
Figure 64 Neurology market estimates and forecasts, 2018-2030 (USD Million)
Figure 65 Cardiology companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 66 Immunology market estimates and forecasts, 2018-2030 (USD Million)
Figure 67 Others market estimates and forecasts, 2018-2030 (USD Million)
Figure 68 Regulatory Affairs Market: Product stage outlook key takeaways
Figure 69 Regulatory Affairs Market: Product stage movement analysis
Figure 70 Preclinical market estimates and forecasts, 2018-2030 (USD Million)
Figure 71 Clinical studies market estimates and forecasts, 2018-2030 (USD Million)
Figure 72 Pre-Market Approval (PMA) companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 73 Immunology market estimates and forecasts, 2018-2030 (USD Million)
Figure 74 Regulatory Affairs Market: Type outlook key takeaways
Figure 75 Regulatory Affairs Market: Type movement analysis
Figure 76 In-house market estimates and forecasts, 2018-2030 (USD Million)
Figure 77 Outsourced market estimates and forecasts, 2018-2030 (USD Million)
Figure 78 Regulatory Affairs Market: Company size outlook key takeaways
Figure 79 Regulatory Affairs Market: Company size movement analysis
Figure 80 Small market estimates and forecasts, 2018-2030 (USD Million)
Figure 81 Medium market estimates and forecasts, 2018-2030 (USD Million)
Figure 82 Large market estimates and forecasts, 2018-2030 (USD Million)
Figure 83 Regulatory Affairs Market: End-use outlook key takeaways
Figure 84 Regulatory Affairs Market: End-use movement analysis
Figure 85 Medical device companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 86 Pharmaceutical companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 87 Biotechnology companies market estimates and forecasts, 2018-2030 (USD Million)
Figure 88 Regional marketplace outlook, 2024 & 2030 (USD Million)
Figure 89 Regional marketplace: Key takeaways
Figure 90 North America Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 91 Key country dynamics
Figure 92 U.S. Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 93 Key country dynamics
Figure 94 Canada Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 95 Key country dynamics
Figure 96 Mexico Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 97 Key country dynamics
Figure 98 Europe Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 99 Key country dynamics
Figure 100 UK regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 101 Key country dynamics
Figure 102 Germany Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 103 Key country dynamics
Figure 104 France Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 105 Key country dynamics
Figure 106 Italy Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 107 Key country dynamics
Figure 108 Spain Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 109 Key country dynamics
Figure 110 Denmark Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 111 Key country dynamics
Figure 112 Norway Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 113 Key country dynamics
Figure 114 Netherlands Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 115 Key country dynamics
Figure 116 Switzerland Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 117 Key country dynamics
Figure 118 Sweden Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 119 Asia Pacific Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 120 Key country dynamics
Figure 121 Japan Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 122 Key country dynamics
Figure 123 China Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 124 Key country dynamics
Figure 125 India Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 126 Key country dynamics
Figure 127 Australia Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 128 Key country dynamics
Figure 129 South Korea Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 130 Key country dynamics
Figure 131 Thailand Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 132 Key country dynamics
Figure 133 Indonesia Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 134 Key country dynamics
Figure 135 Taiwan Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 136 Key country dynamics
Figure 137 Malaysia Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 138 Key country dynamics
Figure 139 Singapore Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 140 Latin America Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 141 Key country dynamics
Figure 142 Brazil Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 143 Key country dynamics
Figure 144 Argentina Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 145 Key country dynamics
Figure 146 Colombia regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 147 Key country dynamics
Figure 148 Chile regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 149 Key country dynamics
Figure 150 MEA Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 151 Key country dynamics
Figure 152 South Africa Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 153 Key country dynamics
Figure 154 Saudi Arabia Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 155 Key country dynamics
Figure 156 UAE Regulatory Affairs Market estimates and forecasts, 2018-2030 (USD Million)
Figure 157 Key country dynamics
Figure 158 Egypt regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 159 Key country dynamics
Figure 160 Israel regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 161 Key country dynamics
Figure 162 Kuwait regulatory affairs market estimates and forecasts, 2018-2030 (USD Million)
Figure 163 Market participant categorization
Figure 164 Regulatory affairs market share/assessment analysis, 2024
Figure 165 Strategic framework
Companies Mentioned
The companies profiled in this Regulatory Affairs market report include:- Accell Clinical Research, LLC
- Genpact
- Criterium, Inc.
- ICON plc
- iuvo BioScience, LLC.
- WuXi AppTec
- Medpace
- Charles River Laboratories.
- Laboratory Corporation of America Holdings
- Parexel International (MA) Corporation
- Freyr
- AmerisourceBergen
- NDA Group AB
- Pharmexon
- Qvigilance
- BlueReg
- Cambridge Regulatory Services
- VCLS
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 16.43 Billion |
| Forecasted Market Value ( USD | $ 27.18 Billion |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |