Epilepsy is second only to stroke as a common chronic neurological disorder characterized by excessive neuronal discharge in the brain resulting in recurrent, seizure and transient central nervous system malfunctions. Epilepsy occurs in people of any age, region, and race, especially in children and adolescents.
The prevalence of epilepsy in China is approximately 7‰, based on which the number of people with epilepsy in China is estimated to be no less than 9 million. More than 60% of Chinese epilepsy patients are children and adolescents, and because of the long and recurrent course of epilepsy, the physical and mental health of patients is seriously threatened, which brings a heavy burden to families and society. In recent years, as China's population ages, the incidence of cerebrovascular disease, dementia, and neurodegenerative diseases has increased. Particularly, the incidence of epilepsy has been on the rise in the elderly population over the age of 50. Approximately there are 400,000 new cases in China each year. Since the number of people with epilepsy is still growing at a faster rate each year, individuals, families and society will be seriously affected.
At present, epilepsy is mainly treated with medication, and if epilepsy patients use medication in a standardized way, about 70% of them can have their condition well controlled.
Levetiracetam was first developed by UCB under the trade name of Keppra®. As a first-line drug in the field of epilepsy treatment, levetiracetam was first marketed in the United States in 1999 in tablet form, and the extended-release tablet form was approved by the FDA in 2008, which is now widely regarded as the gold standard drug for epilepsy treatment.
Keppra was approved for marketing in China in November 2006. In 2009, after the expiration of the patent, generic drugs by Chinese companies were marketed, but Keppra still occupied a major market share. The dosage forms of levetiracetam include tablet, oral solution and injection. Among them, levetiracetam tablets have the main market share and the fiercest market competition with increasing generic drugs.
According to the publisher’s market research, the sales value of levetiracetam in the Chinese market continued to rise from 2016 to 2019. In 2020, the COVID-19 outbreak prevented Chinese healthcare facilities from functioning properly. And as the various dosage forms of levetiracetam are prescription drugs in China, the total sales value of levetiracetam declined to approximately CNY319 million (USD49.1 million) with a CAGR of approximately 2.3% from 2016 to 2020.
The publisher expects that from 2021 to 2025, China’s levetiracetam market will show a recovery growth in both sales volume and value due to the effective control of COVID-19 and the resumption of proper operation of healthcare facilities. The number of patients with epilepsy in China will continue to rise because of factors such as the accelerated pace of life and work and poor lifestyle behaviors like late nights, alcohol abuse, and the increasingly aging population, driving up both sales volume and value of levetiracetam in the Chinese market.
Topics Covered:
- Impact of COVID-19 on China’s Levetiracetam Market
- Development Environment of Levetiracetam in China
- Sales Volume of Levetiracetam in China
- Sales Volume and Value of Levetiracetam in China by Region
- Major Levetiracetam Manufacturers in China and Their Market Shares
- Sales Price of Levetiracetam in China
- Prospects of China’s Levetiracetam Market, 2021-2025
Table of Contents
1 Relevant Concepts of Levetiracetam
1.1 Indications of Levetiracetam1.2 Development of China’s Levetiracetam Market
1.3 Governmental Approval of Levetiracetam in China
1.4 The Impact of COVID-19 on China’s Levetiracetam Market
2 Sales of Levetiracetam in China, 2016-2020
2.1 Sales Value2.1.1 Sales Value in China
2.1.2 Sales Value in China by Region
2.2 Sales Volume
2.2.1 Sales Volume in China
2.2.2 Sales Volume in China by Region
2.3 Sales of Levetiracetam in China by Dosage Form, 2016-2020
2.3.1 Tablets
2.3.2 Concentrated Solution for Injection
2.3.3 Other Dosage Forms
3 Analysis of Major Levetiracetam Manufacturers in China, 2020
3.1 Analysis of Market Share3.1.1 Market Share of Manufacturers by Sales Value
3.1.2 Market Share of Manufacturers by Sales Volume
3.2 UCB Pharma SA
3.2.1 Company Profile
3.2.2 Sales of Levetiracetam in China
3.3 Zhejiang Jingxin Pharmaceutical Co. Ltd.
3.3.1 Company Profile
3.3.2 Sales of Levetiracetam in China
3.4 Zhejiang Apeloa Kangyu Bio-pharmaceutical Co. Ltd.
3.5 Chengdu Tiantai Mount pharmaceutical Co., Ltd.
3.6 Patheon Italia SpA
4 Sales Price of Levetiracetam of Different Companies in China, 2020-2021
4.1 UCB Pharma SA (Keppra®)4.2 Zhejiang Jingxin Pharmaceutical Co. Ltd. (Jiyike®)
4.3 Zhejiang Apeloa Kangyu Bio-pharmaceutical Co. Ltd. (YOUSEMADE®)
4.4 Chengdu Tiantai Mount pharmaceutical Co., Ltd. (PuNingKe®)
4.5 Patheon Italia SpA (Keppra®)
5 Prospects of China’s Levetiracetam Market, 2021-2025
5.1 Influencing Factors for the Market Development5.1.1 The Impact of COVID-19 on the Market
5.1.2 Market Drivers and Opportunities
5.1.3 Market Threats and Challenges
5.2 Forecast on Market Size
5.3 Forecast on Market Trend
List of Charts
- Chart Patent Information of Levetiracetam in China
- Chart Sales Value of Levetiracetam in China, 2016-2020
- Chart Sales Volume of Levetiracetam in China, 2016-2020
- Chart Sales Volume of Levetiracetam in China by Region, 2016-2020
- Chart Sales Value of Levetiracetam in China by Region, 2016-2020
- Chart Sales Volume and Value of Levetiracetam Injection in China, 2016-2020
- Chart Market Share of Levetiracetam Manufacturers in China by Sales Value, 2016-2020
- Chart Sales Value and Volume of Levetiracetam (of UCB Pharma SA) in China, 2016-2020
- Chart Sales Value and Volume of Levetiracetam (of Zhejiang Jingxin Pharmaceutical Co. Ltd.) in China, 2016-2020
- Chart Sales Value and Volume of Levetiracetam (of Zhejiang Apeloa Kangyu Bio-pharmaceutical Co. Ltd.) in China, 2016-2020
- Chart Sales Price of Keppra (Levetiracetam of UCB Pharma SA) in China by Region, 2020
- Chart Sales Price of Jiyike (Levetiracetam of Zhejiang Jingxin Pharmaceutical Co. Ltd.) in China by Region, 2020
- Chart Sales Price of YOSEMADE (Levetiracetam of Zhejiang Apeloa Kangyu Bio-pharmaceutical Co. Ltd.) in China by Region, 2020
- Chart Forecast on Sales Value of Levetiracetam in China, 2021-2025
- Chart Forecast on Sales Volume of Levetiracetam in China, 2021-2025
Companies Mentioned
- UCB Pharma SA (Keppra®)
- Zhejiang Jingxin Pharmaceutical Co. Ltd. (Jiyike®)
- Zhejiang Apeloa Kangyu Bio-pharmaceutical Co. Ltd. (YOUSEMADE®)
- Chengdu Tiantai Mount pharmaceutical Co., Ltd. (PuNingKe®)
- Patheon Italia SpA (Keppra®)
Methodology
Background research defines the range of products and industries, which proposes the key points of the research. Proper classification will help clients understand the industry and products in the report.
Secondhand material research is a necessary way to push the project into fast progress. The analyst always chooses the data source carefully. Most secondhand data they quote is sourced from an authority in a specific industry or public data source from governments, industrial associations, etc. For some new or niche fields, they also "double-check" data sources and logics before they show them to clients.
Primary research is the key to solve questions, which largely influence the research outputs. The analyst may use methods like mathematics, logical reasoning, scenario thinking, to confirm key data and make the data credible.
The data model is an important analysis method. Calculating through data models with different factors weights can guarantee the outputs objective.
The analyst optimizes the following methods and steps in executing research projects and also forms many special information gathering and processing methods.
1. Analyze the life cycle of the industry to understand the development phase and space.
2. Grasp the key indexes evaluating the market to position clients in the market and formulate development plans
3. Economic, political, social and cultural factors
4. Competitors like a mirror that reflects the overall market and also market differences.
5. Inside and outside the industry, upstream and downstream of the industry chain, show inner competitions
6. Proper estimation of the future is good guidance for strategic planning.

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 40 |
| Published | September 2021 |
| Forecast Period | 2016 - 2025 |
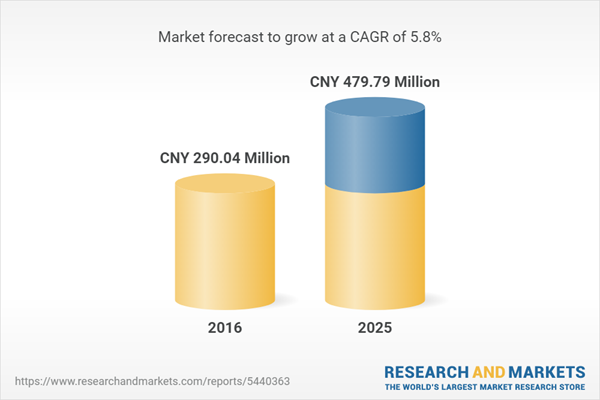
| Estimated Market Value ( CNY | CNY 290.04 Million |
| Forecasted Market Value ( CNY | CNY 479.79 Million |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | China |
| No. of Companies Mentioned | 5 |