Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, a major obstacle hindering market growth is the intricate nature of regulatory validation for these computational models. Demonstrating the reliability of virtual simulations to meet strict regulatory requirements remains a complex task, primarily because industry frameworks for confirming the predictive accuracy of these models against actual human biological data are still evolving. This lack of mature verification standards creates a significant barrier to widespread commercial adoption.
Market Drivers
The rising expense and intricacy of conventional clinical trials are the main factors driving the pharmaceutical sector toward in silico methods. As biological targets grow more complex, the financial strain of running extensive physical trials has become unmanageable, necessitating virtual cohorts that can evaluate efficacy prior to human involvement. This need to shorten development cycles is highlighted by recent advancements in generative workflows that enable companies to skip prolonged traditional steps. For instance, Insilico Medicine reported in a March 2024 press release regarding a study in 'Nature Biotechnology' that their AI-powered platform identified a therapeutic candidate in roughly 18 months, a timeframe notably shorter than the standard multi-year preclinical period, demonstrating how computational models can lower the risk of late-stage failures and improve resource management.Furthermore, rapid developments in artificial intelligence and high-performance computing are fueling market growth by improving the predictive accuracy of virtual simulations. Moving from static models to dynamic, generative algorithms enables the creation of highly precise digital twins that mimic human physiological reactions. In May 2024, Google DeepMind announced that their upgraded 'AlphaFold 3' model secured a 50% increase in prediction accuracy for protein-molecule interactions, offering the detailed data needed for dependable virtual testing. This technological progress has prompted significant investment to scale these capabilities, as seen in April 2024 when Xaira Therapeutics launched with over USD 1 billion in committed capital to embed these advanced computational techniques into the entire drug development lifecycle.
Market Challenges
The intricate process of obtaining regulatory validation for computational models poses a significant barrier to the growth of the Global In Silico Clinical Trials Market. Although simulation technologies promise theoretical efficiency, proving the credibility of these virtual approaches to meet rigorous safety standards remains challenging. Regulatory authorities demand solid proof that computer models can precisely forecast human biological responses, yet the sector currently lacks fully developed, standardized frameworks to consistently prove this reliability. As a result, pharmaceutical developers encounter considerable uncertainty and the threat of rejection during approval procedures, which deters the financial commitment needed to shift from conventional techniques to virtual patient cohorts.This challenge is further compounded by the struggle to access high-quality real-world data required to verify these models. Confirming predictive accuracy necessitates extensive benchmarking against exact human biological datasets, which are frequently fragmented or inaccessible. According to the Pistoia Alliance in 2024, 52% of life science professionals identified low-quality and poorly curated datasets as the primary obstacle to implementing these sophisticated computational technologies. This lack of reliable validation data directly worsens regulatory difficulties, hindering companies from compiling the strong evidence dossiers required for market approval and delaying the commercial uptake of in silico trials.
Market Trends
The creation of high-fidelity digital twins for virtual patient cohorts is fundamentally transforming regulatory submissions by enabling developers to model drug performance across varied physiological populations without the need for human recruitment. This shift is especially apparent in the broad use of physiologically based pharmacokinetic modeling, which creates virtual cohorts to forecast drug interactions in specific demographics, such as pediatric patients or individuals with organ impairment. Consequently, pharmaceutical firms are increasingly utilizing these simulations to obtain label approvals directly, effectively substituting for certain in vivo studies. As noted by Certara in their September 2024 'Simcyp Consortium Celebrates 25th Anniversary' announcement, their platform's simulations have successfully guided dosing decisions for over 375 label claims covering 115 different drugs, replacing physical clinical trials.Concurrently, the use of virtual control arms is redefining trial design by allowing sponsors to replace conventional placebo groups with synthetic data generated from historical clinical records. This method is gaining considerable momentum in oncology and rare disease research, where enrolling enough participants for standard control arms is often ethically difficult or logistically impractical. By leveraging extensive historical datasets, researchers can build statistically sound external comparators that uphold scientific rigor while significantly lowering patient enrollment needs. According to Medidata's August 2024 report on 'The Regulatory Grade External Control Arm', their proprietary database for creating these synthetic arms now includes historical clinical trial data from more than 33,000 trials and 10 million patients, offering the detailed evidence needed to sustain these hybrid trial models.
Key Players Profiled in the In Silico Clinical Trials Market
- Dassault Systemes
- Certara
- Insilico Medicine
- GNS Healthcare
- Siemens Healthineers
- Evidera
- Novadiscovery
- Simulations Plus
- Genentech
- Physiomics
Report Scope
In this report, the Global In Silico Clinical Trials Market has been segmented into the following categories:In Silico Clinical Trials Market, by Industry:
- Medical Devices v/s Pharmaceuticals
In Silico Clinical Trials Market, by Therapeutic Area:
- Oncology
- Neurology
- Cardiology
- Infectious Diseases
- Orthopedic
- Dermatology
- Others
In Silico Clinical Trials Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global In Silico Clinical Trials Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this In Silico Clinical Trials market report include:- Dassault Systemes
- Certara
- Insilico Medicine
- GNS Healthcare
- Siemens Healthineers
- Evidera
- Novadiscovery
- Simulations Plus
- Genentech
- Physiomics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
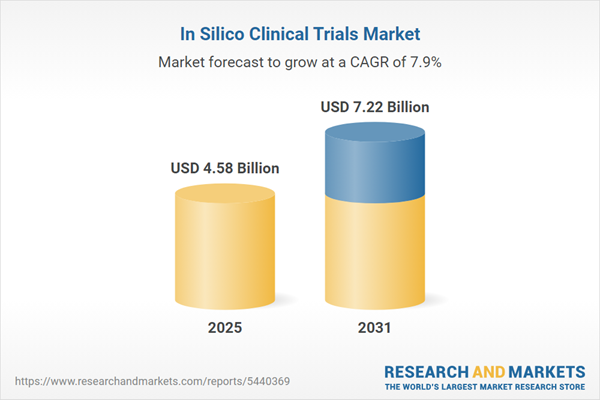
| Estimated Market Value ( USD | $ 4.58 Billion |
| Forecasted Market Value ( USD | $ 7.22 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |