Atherectomy Devices Market Trends:
Rising Cases of Peripheral Artery Disease (PAD)The increasing cases of PAD at a global level due to ageing population and the rise in diabetes and obesity is leading the atherectomy device market growth. Approximately, PAD affects 8 to 12 million people in the United States, especially in those over 50. Out of these, 6.5 million over the age of 40 are diagnosed with PAD. Atherectomy devices provide a minimally invasive approach to remove arterial plaque as it is an important alternative in vascular interventions. The device enhances blood flow and reduces symptoms such as leg pain and fatigue. By directly cutting away or vaporizing the plaque, these devices help restore arterial patency, significantly improving patient outcomes in individuals with severe forms of PAD.
Technological Advancements
Major atherectomy devices innovations include the appearance of directionally controlled devices and high-frequency rotational technologies, which have continued to improve the effectiveness and safety of these devices. For instance, a 2020 study suggests that orbital atherectomy (which sands down plaque) effectively opens up blocked leg arteries 90 percent of the time, while laser atherectomy achieves similarly improved blood flow 76 percent of the time. These improvements in patient outcome and surgeon preference for these devices are propelling the growth of the atherectomy devices. In a multicenter study of 172 patients, Jetstream use had a 99% device success, and six-month and 12-month clinically-driven, target-lesion revascularization rates of 15% and 26%, respectively; with a one-year restenosis rate of 38% based on duplex imaging.Expansion into Emerging Markets
Prominent key players are focusing on emerging economies where healthcare infrastructure development and growing healthcare spending is focused, thereby creating new opportunities for the key players in the market. For instance, the emerging markets of Brazil, Russia, India, China, and South Africa (BRICS) are increasingly shaping the landscape of the global health sector demand and supply for medical goods and services. Health spending as a percentage of GDP in 2030 is projected as - Brazil, 8.4%; Russia, 5.2%; India, 3.5%; China, 5.9%; South Africa, 10.4%. The penetration into these markets is enabled by realization of more advanced treatment alternatives, thus expanding the global footprint of atherectomy devices.Atherectomy Devices Market Segmentation:
The publisher provides an analysis of the key trends in each segment of the market, along with forecasts at the global, regional and country levels for 2025-2033. Our report has categorized the market based on product, application, and end user.Breakup by Product:
- Directional Atherectomy Devices
- Orbital Atherectomy Devices
- Photo-Ablative Atherectomy Devices
- Rotational Atherectomy Devices
- Support Devices
Directional Atherectomy Devices accounts for the majority of the market share
The report has provided a detailed breakup and analysis of the market based on the product. This includes directional atherectomy devices, orbital atherectomy devices, photo-ablative atherectomy devices, rotational atherectomy devices, and support devices. According to the report, directional atherectomy devices represented the largest segment.Directional atherectomy devices are created to precisely eliminate atherosclerotic plaque from obstructed arteries. These instruments provide the possibility of accurate control, which reduces vessel wall trauma, an important aspect for patient safety and recovery. Their ability to treat complex lesions in multiple vessel sizes makes them to be favoured by vascular specialists leading to the growth of the product segment. The capability of the devices to collect and remove plaque during the procedure sets these devices apart and is in a number of clinical situations preferable.
Breakup by Application:
- Peripheral Vascular
- Cardiovascular
- Neurovascular
Peripheral Vascular accounts for the majority of the market share
The report has provided a detailed breakup and analysis of the market based on the application. This includes peripheral vascular, cardiovascular, and neurovascular. According to the report, peripheral vascular represented the largest segment.The use of atherectomy devices in the treatment of peripheral vascular diseases is important because of the growing prevalence of PAD. Such devices play a key role in reestablishing the blood flow in leg arteries thus help to relieve the symptoms such as pain and mobility problems. The demand in this segment is determined by minimally invasive character of the procedure which allows short recovery period and lower risks of complications in comparison to conventional surgery making patient outcomes and treatment efficiencies higher.
Breakup by End User:
- Hospitals
- Ambulatory Surgery Centers
- Research Laboratories and Academic Institutes
Hospitals accounts for the majority of the market share
The report has provided a detailed breakup and analysis of the market based on the end user. This includes hospitals, ambulatory surgery centers, and research laboratories and academic institutes. According to the report, hospitals represented the largest segment.Hospitals are the main users of atherectomy devices because these procedures are very complex and need advanced infrastructure and highly trained healthcare professionals. The growth of the segment is followed by the growth of the number of vascular surgeries conducted in hospitals, fueled by investments into advanced technologies and in the training programs of health providers. Ability of hospitals to offer all types of care including post-operative requirements also helps in making them the dominant subject in the market.
Breakup by Region:
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
North America dominates the market
The report has also provided a comprehensive analysis of all the major markets which include North America (United States, Canada); Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others); Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others); Latin America (Brazil, Mexico, Others), and Middle East and Africa. According to the report, North America was the largest market for atherectomy devices.North America is the largest market for atherectomy because of high prevalence of cardiovascular diseases, developed healthcare system and the key market players in the region. The United States is the biggest market in this region because of the favourable reimbursement scenarios, clinical trials, and solid governmental support for research and development in advanced medical devices. The commitment of the region to application of new healthcare solutions is driving the market growth.
Competitive Landscape:
- The market research report has also provided a comprehensive analysis of the competitive landscape in the market. Detailed profiles of all major companies have been provided. Some of the major market players in the atherectomy devices market include Abbott Laboratories, Avinger, B. Braun Melsungen AG, Biomerics, Biotronik, Boston Scientific Corporation, C.R. Bard (Becton, Dickinson and Company), Cardinal Health, Koninklijke Philips N.V., Medtronic, Minnetronix Inc., Straub Medical AG (Becton, Dickinson and Company), and Terumo Corporation.
- There is a dynamic market in atherectomy devices with the use of evolving strategies by major players in pursuit to grow their market share and to satisfy the increasing demand for minimally invasive surgery alternatives. One of these approaches is technical oriented, which is to say that the companies invest significantly in R&D to improve their devices that become therefore more efficient and safer. The second common event is strategic alliances and collaborations that support knowledge sharing and better distribution channels. In addition, mergers and acquisitions are common enabling companies to expand their product portfolios and geographical coverage. Approvals by regulatory bodies play an important role here as the companies are trying to meet tough norms that guarantee the efficiency and safety of their products in many markets.
Key Questions Answered in This Report
1. What was the size of the global atherectomy devices market in 2024?2. What is the expected growth rate of the global atherectomy devices market during 2025-2033?
3. What has been the impact of COVID-19 on the global atherectomy devices market?
4. What are the key factors driving the global atherectomy devices market?
5. What is the breakup of the global atherectomy devices market based on the product?
6. What is the breakup of the global atherectomy devices market based on the application?
7. What is the breakup of the global atherectomy devices market based on the end user?
8. What are the key regions in the global atherectomy devices market?
9. Who are the key players/companies in the global atherectomy devices market?
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Avinger
- B. Braun Melsungen AG
- Biomerics
- Biotronik
- Boston Scientific Corporation
- C.R. Bard (Becton
- Dickinson and Company)
- Cardinal Health
- Koninklijke Philips N.V.
- Medtronic
- Minnetronix Inc.
- Straub Medical AG (Becton
- Dickinson and Company)
- Terumo Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 137 |
| Published | August 2025 |
| Forecast Period | 2024 - 2033 |
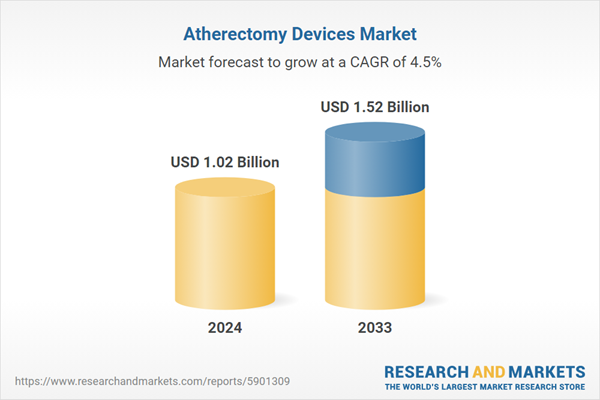
| Estimated Market Value ( USD | $ 1.02 Billion |
| Forecasted Market Value ( USD | $ 1.52 Billion |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |