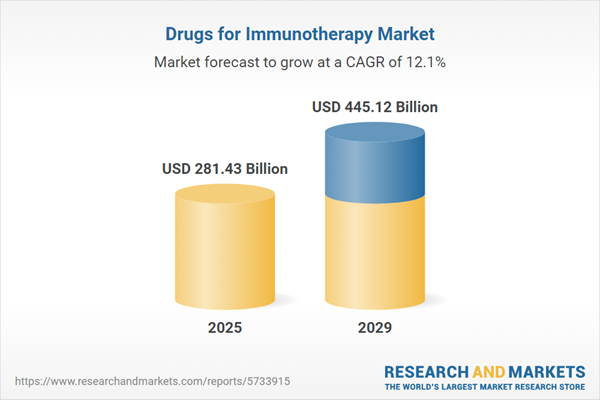
The drugs for immunotherapy market size has grown rapidly in recent years. It will grow from $255.14 billion in 2024 to $281.43 billion in 2025 at a compound annual growth rate (CAGR) of 10.3%. The growth in the historic period can be attributed to the rising incidence of cancer, unmet medical needs, cancer immunotherapy awareness, higher adoption rates of immunotherapeutic approaches, and rising investment by pharmaceutical giants.
The drugs for immunotherapy market size is expected to see rapid growth in the next few years. It will grow to $445.12 billion in 2029 at a compound annual growth rate (CAGR) of 12.1%. The growth in the forecast period can be attributed to combination therapies, personalized medicine, global expansion, biomarker-driven therapies, and increasing clinical trials. Major trends in the forecast period include advancements in immunology, advancements in car-t cell therapies, targeted immunotherapies, immunotherapy combinations, and neoantigen vaccines.
The burgeoning prevalence of cancer worldwide stands as the primary impetus behind the escalating demand for drugs in the immunotherapy market. The proliferation of cancer cases, attributed to factors like obesity, smoking, alcohol consumption, and dietary habits, is projected to soar significantly. Reports from Macmillan Cancer Support in October 2022 estimated a steep rise in cancer cases, predicting 3.5 million by 2025, 4 million by 2030, and 5.3 million by 2040. With breast, lung, colon, rectum, and prostate cancers ranking as the most widespread types, lifestyle elements such as tobacco use, high BMI, limited fruit and vegetable intake, alcohol consumption, and lack of exercise contribute to roughly one-third of cancer-related deaths. This escalation in cancer incidence is set to drive up the demand for immunotherapy drugs, consequently fueling market growth.
The rising healthcare expenditure is expected to drive the growth of the immunotherapy drugs market in the future. Healthcare expenditure encompasses the total amount spent on healthcare goods and services within a specific region or country over a defined period. This includes expenses for doctor visits, hospital stays, surgeries, diagnostic tests, and treatments. Financial resources allocated for healthcare come from individuals, healthcare providers, insurance companies, and government agencies to cover costs related to immunotherapy drugs and treatments. For instance, a report from the Centers for Medicare & Medicaid Services in December 2022 indicated that U.S. healthcare spending increased by 4.1% to reach $4.5 trillion in 2022, surpassing the 3.2% growth seen in 2021. As a result, the rise in healthcare expenditure is driving the growth of the drugs for immunotherapy market.
Prominent entities within the immunotherapy drugs market are strategically focused on innovating novel products such as Keytruda (pembrolizumab), aimed at delivering dependable services to consumers. Keytruda, an immune checkpoint inhibitor categorized under immunotherapy, was launched by Merck & Co., Inc. in January 2023. Approved by the US Food and Drug Administration, this breakthrough therapy assists the immune system in combating cancer cells, notably approved as an adjuvant treatment for non-small cell lung cancer (NSCLC). This development stands as a substantial leap in cancer treatment, poised to positively impact the healthcare industry's landscape.
Manufacturers of drugs for immunotherapy market are increasingly collaborating or partnering with other companies to share technology, resources, and product knowledge and expand the business. For example, Illumina Inc. and Bristol-Myers Squibb (BMS) collaborated to utilize Illumina’s next-generation sequencing (NGS) technology to develop and commercialize in-vitro diagnostic (IVD) assays in support of Bristol-Myers Squibb's oncology portfolio. Allogene Therapeutics Inc. and Pfizer Inc. entered into an asset contribution agreement to use Pfizer’s portfolio of assets related to allogeneic CAR-T therapy.
In December 2024, Pfizer Inc., a pharmaceutical company based in the United States, completed the acquisition of Seagen for $43 billion. This acquisition is intended to enhance Pfizer's oncology portfolio by incorporating advanced immunotherapy treatments that utilize Seagen's innovative antibody-drug conjugates (ADCs) for targeted cancer therapy. Seagen Inc. is a biotechnology firm located in the U.S. that focuses on the production of antibody-drug conjugates (ADCs).
Immunotherapy drugs constitute a category of medications designed to modulate the immune system, either by enhancing or suppressing it, to bolster the body's defense against cancer, infections, and various ailments. This approach to cancer treatment harnesses the body's immune response to combat the disease.
The primary classes of immunotherapy drugs encompass monoclonal antibodies, interferons, interleukins, vaccines, checkpoint inhibitors, and other specialized treatments. Monoclonal antibodies, for instance, are produced by replicating a single white blood cell, allowing the lineage of each antibody to be traced back to its originating parent cell. These drugs find application across diverse medical domains such as cancer treatment, autoimmune conditions, inflammatory diseases, and combating infections. They are employed across various healthcare settings including hospitals, clinics, ambulatory surgical centers, and other healthcare facilities.
The drugs for immunotherapy market research report is one of a series of new reports that provides drugs for immunotherapy market statistics, including drugs for immunotherapy industry global market size, regional shares, competitors with drugs for immunotherapy market share, detailed drugs for immunotherapy market segments, market trends and opportunities, and any further data you may need to thrive in the drugs for immunotherapy industry. This drug for immunotherapy market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Major companies operating in the drugs for immunotherapy market include F. Hoffmann-La Roche Ltd., Novartis AG, Amgen Inc., AbbVie Inc., Merck & Co. Inc., GlaxoSmithKline plc, Eli Lilly and Company, Bristol-Myers Squibb, AstraZeneca plc, Johnson & Johnson, UbiVac, Pfizer Inc., Sanofi SA, Bayer AG, Celgene Corporation, Incyte Corporation, Juno Therapeutics Inc., Bluebird bio, Celldex Therapeutics, BeiGene, BioNTech AG, CytomX Therapeutics, Eisai Co. Ltd., Flexus Biosciences Inc., Genscript Biotech Corporation, Gilead Sciences Inc., Harpoon Therapeutics Inc., Heptares Therapeutics Ltd., Ignyta Inc., Immatics Biotechnologies, Jounce Therapeutics Inc.
North America was the largest region in the immunotherapy market in 2024. Middle East is expected to be the fastest growing region in the drug for immunotherapy market report during the forecast period. The regions covered in the drugs for immunotherapy market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa. The countries covered in the drugs for immunotherapy market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
The drugs for immunotherapy market consist of sales of cytokines, CAR T-cell therapy, and cancer treatment vaccines. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Drugs for Immunotherapy Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on drugs for immunotherapy market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for drugs for immunotherapy ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The drugs for immunotherapy market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Monoclonal Antibodies; Interferons; Interleukins; Vaccines; Checkpoint Inhibitors; Other Types2) By Therapy Area: Cancer; Autoimmune & Inflammatory Diseases; Infectious Diseases; Other Therapy Areas
3) By End User: Hospitals & Clinics; Ambulatory Surgical Centers; Other End Users
Subsegments:
1) By Monoclonal Antibodies: Naked Monoclonal Antibodies; Conjugated Monoclonal Antibodies; Bispecific Monoclonal Antibodies2) By Interferons: Interferon Alfa; Interferon Beta; Interferon Gamma
3) By Interleukins: Interleukin-2 (IL-2); Interleukin-6 (IL-6); Interleukin-12 (IL-12)
4) By Vaccines: Preventive Vaccines; Therapeutic Vaccines
5) By Checkpoint Inhibitors: PD-1 Inhibitors; PD-L1 Inhibitors; CTLA-4 Inhibitors
6) By Other Types: Cancer Cell Therapies; Cytokine Therapy; T-Cell Receptor Therapy
Key Companies Mentioned: F. Hoffmann-La Roche Ltd.; Novartis AG; Amgen Inc.; AbbVie Inc.; Merck & Co. Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Drugs for Immunotherapy market report include:- F. Hoffmann-La Roche Ltd.
- Novartis AG
- Amgen Inc.
- AbbVie Inc.
- Merck & Co. Inc.
- GlaxoSmithKline plc
- Eli Lilly and Company
- Bristol-Myers Squibb
- AstraZeneca plc
- Johnson & Johnson
- UbiVac
- Pfizer Inc.
- Sanofi SA
- Bayer AG
- Celgene Corporation
- Incyte Corporation
- Juno Therapeutics Inc.
- Bluebird bio
- Celldex Therapeutics
- BeiGene
- BioNTech AG
- CytomX Therapeutics
- Eisai Co. Ltd.
- Flexus Biosciences Inc.
- Genscript Biotech Corporation
- Gilead Sciences Inc.
- Harpoon Therapeutics Inc.
- Heptares Therapeutics Ltd.
- Ignyta Inc.
- Immatics Biotechnologies
- Jounce Therapeutics Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 281.43 Billion |
| Forecasted Market Value ( USD | $ 445.12 Billion |
| Compound Annual Growth Rate | 12.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 32 |