Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, market expansion faces a substantial obstacle due to the prohibitive costs of advanced therapeutic options. The high pricing of recombinant factors and innovative therapies creates significant accessibility barriers, especially in low- and middle-income countries where reimbursement structures are frequently inadequate. This economic disparity limits the addressable market within developing regions and remains a critical hurdle for manufacturers seeking to extend their global footprint.
Market Drivers
The introduction of novel gene therapies and curative solutions is a major driver transforming the global haemophilia treatment landscape, shifting the focus from chronic disease management to potential one-time cures. This evolution is defined by the approval of high-value treatments offering long-term bleed protection through a single administration, which subsequently alters payer models and long-term revenue outlooks. A key market development underscores this value shift; as reported by Fierce Pharma in April 2024 in the article 'Pfizer scores FDA nod for hemophilia B gene therapy', the newly approved gene therapy Beqvez launched with a list price of $3.5 million. Despite the substantial upfront investment, such therapies are attracting significant interest from healthcare systems seeking to reduce the lifetime economic burden associated with severe bleeding disorders.Simultaneously, the rapid adoption of non-factor replacement therapies is revolutionizing standard prophylactic care by lowering the treatment burden for patients. The market is witnessing a decisive move from intravenous clotting factors to subcutaneous bispecific antibodies that provide reduced dosing frequency and consistent efficacy. This trend is supported by strong financial performance; according to Roche’s 'Finance Report 2023' released in February 2024, full-year sales of the non-factor therapy Hemlibra reached CHF 4.14 billion, highlighting its dominance in prophylactic settings. While non-factor agents are expanding, overall therapeutic demand remains massive; the World Federation of Hemophilia’s 'Report on the Annual Global Survey 2023', published in October 2024, indicated that global consumption of Factor VIII concentrates was approximately 9.4 billion IU, emphasizing the sustained scale of worldwide therapeutic requirements.
Market Challenges
The prohibitive costs associated with advanced therapeutic options constitute a formidable barrier to the growth of the global haemophilia treatment market. The high price tags on recombinant clotting factors and novel gene therapies create a steep financial threshold that many healthcare systems in developing regions cannot overcome. In nations where public reimbursement frameworks are insufficient or nonexistent, these life-saving treatments remain largely inaccessible to the majority of the patient population. This economic disconnect severely restricts the commercial reach of pharmaceutical manufacturers, compelling them to rely heavily on saturated high-income markets while leaving substantial demand in emerging economies unaddressed due to affordability constraints.The consequences of this economic disparity are clearly visible in the uneven global distribution of treatment products. Data from the World Federation of Hemophilia indicates that in 2023, high-income countries were responsible for 61% of total global Factor VIII usage, whereas low-income countries contributed a mere 0.3% to global consumption. This stark imbalance demonstrates that, despite rising diagnosis rates, the market’s potential is artificially capped by the inability of lower-income nations to purchase premium therapeutics. Consequently, the industry struggles to convert the growing global patient pool into tangible revenue, thereby hampering overall market expansion.
Market Trends
The widespread adoption of Extended Half-Life Factor Concentrates is actively reshaping the competitive dynamics of the haemophilia sector by renewing the relevance of factor replacement therapies amidst the rise of non-factor alternatives. This trend involves the uptake of next-generation fusion proteins that significantly extend the circulating half-life of Factor VIII, enabling patients to maintain high sustained factor activity with reduced injection frequency. The immediate commercial impact of this shift is evident in the rapid market penetration of recently approved agents that offer once-weekly dosing while preserving peak protection levels. According to Sanofi’s 'Press Release: Sanofi Q3 2024' from October 2024, sales of the extended half-life therapy Altuviiio reached €172 million in the third quarter alone, driven primarily by patients switching from standard factor regimens and other therapeutics.Parallel to the evolution of factor replacement, the advancement in Novel RNA Interference Therapeutics is introducing a distinct mechanism of action that targets the coagulation cascade at a fundamental level. Unlike replacement factors or bispecific antibodies, these investigational therapies utilize small interfering RNA to suppress antithrombin production, effectively rebalancing hemostasis for patients with either Haemophilia A or B, regardless of inhibitor status. This approach aims to provide consistent bleed protection through a substrate-independent pathway, marking a departure from traditional protein replacement strategies. New clinical data supports this mechanism's precision; according to Sanofi’s June 2024 press release regarding ISTH presentations, phase 3 study results demonstrated that maintaining antithrombin activity levels between 15% and 35% via this RNAi therapy resulted in clinically meaningful bleed control for patients.
Key Players Profiled in the Haemophilia Treatment Market
- Bayer AG
- BioMarin Pharmaceutical, Inc.
- CSL Behring LLC
- Kedrion S.p.A
- Novo Nordisk A/S
- Pfizer, Inc.
- Octapharma AG
- Sanofi SA
- Takeda Pharmaceutical Company Limited
- Grifols S.A.
Report Scope
In this report, the Global Haemophilia Treatment Market has been segmented into the following categories:Haemophilia Treatment Market, by Type:
- Haemophilia A
- Haemophilia B
- Haemophilia C
Haemophilia Treatment Market, by Product:
- Recombinant Coagulation Factor Concentrates
- Plasma Derived Coagulation Factor Concentrate
- Desmopressin
- Antifibrinolytics Agents
Haemophilia Treatment Market, by Treatment:
- On-demand and Prophylaxis
Haemophilia Treatment Market, by Therapy:
- Replacement Therapy
- ITI therapy and Gene Therapy
Haemophilia Treatment Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Haemophilia Treatment Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Haemophilia Treatment market report include:- Bayer AG
- BioMarin Pharmaceutical, Inc.
- CSL Behring LLC
- Kedrion S.p.A
- Novo Nordisk A/S
- Pfizer, Inc.
- Octapharma AG
- Sanofi SA
- Takeda Pharmaceutical Company Limited
- Grifols S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
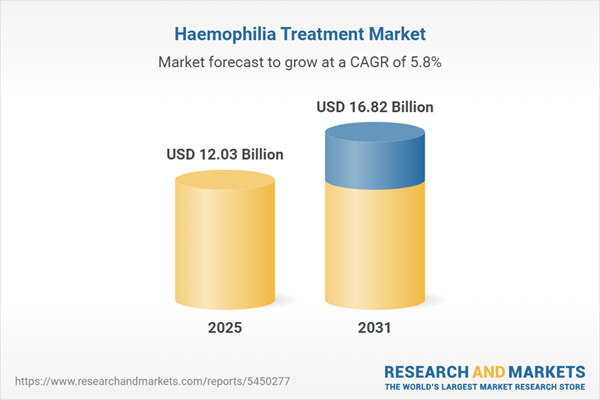
| Estimated Market Value ( USD | $ 12.03 Billion |
| Forecasted Market Value ( USD | $ 16.82 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |