Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative introduction describing how technology, clinical practice evolution, and patient expectations are converging to redefine sleep apnea device strategies
The sleep apnea device landscape sits at the intersection of medical technology innovation, evolving clinical practice, and shifting patient expectations. Advances in device design, algorithmic pressure modulation, and materials science have translated into more comfortable, effective, and adaptable solutions for obstructive and central sleep apnea management. At the same time, care delivery models are decentralizing; telehealth-enabled titration and home-based diagnostics are reducing friction for patients while expanding the addressable population for therapy initiation.Regulatory scrutiny has grown commensurate with technological complexity, prompting manufacturers to prioritize post-market surveillance, cybersecurity hardening, and data interoperability. Payers and providers are increasingly focused on demonstrable adherence and long-term clinical outcomes, which has driven investment in integrated ecosystems that combine machine hardware, masks, humidification components, and cloud-based adherence platforms. As clinical guidelines evolve and health systems emphasize cost-effective chronic disease management, device manufacturers and channel partners must balance innovation with affordability and operational simplicity.
This introduction frames subsequent sections by highlighting the converging forces-clinical demand, regulatory expectation, and distribution transformation-that are reshaping product roadmaps and commercial strategies across the sleep apnea device sector.
A strategic analysis of how device innovation, remote care adoption, and supply chain resilience are collectively reshaping competitive advantage in the sleep apnea sector
The past several years have produced transformative shifts across product engineering, clinical workflows, and supply chain approaches that collectively alter competitive dynamics. Algorithm-driven automatic positive airway pressure systems and dual-level bilevel platforms are moving from feature differentiation to core expectations, as clinicians and patients demand both comfort and objective efficacy. Mask design and humidification integration have evolved in parallel, reducing barriers to adherence and expanding the viable population for home-based therapy initiation.Concurrently, the digital layer-remote monitoring, device connectivity, and algorithmic adherence coaching-has transitioned from optional enhancement to a required component of many payer and health system contracts. Telemedicine adoption has accelerated titration and follow-up pathways, diminishing the reliance on in-clinic visits and enabling earlier intervention. Supply chain resilience has become a strategic priority, with manufacturers diversifying suppliers and nearshoring select production elements to reduce lead-time volatility.
These shifts necessitate that product roadmaps emphasize modularity, serviceability, and data-enabled outcomes reporting. Market participants that successfully integrate advanced device modalities with seamless patient experiences and robust channel strategies will secure differentiated positions in an increasingly outcome-oriented environment.
A rigorous qualitative assessment of how proposed 2025 tariff adjustments may influence sourcing strategies, pricing levers, and manufacturing footprint decisions across the industry
Policy measures such as tariffs and trade policy changes can materially influence sourcing decisions, pricing strategies, and regional manufacturing footprints for medical device firms. Proposed tariff adjustments in 2025 create an imperative for manufacturers to reassess supplier contracts, tariff engineering opportunities, and total landed cost calculations across global value chains. Increased import duties on core machine components or finished units would prompt a re-evaluation of component localization, contract renegotiation, and potential redesign to minimize tariff exposure while preserving clinical performance.Manufacturers with vertically integrated supply chains or diversified regional manufacturing capabilities will be better positioned to absorb or mitigate tariff-induced cost fluctuations. Those dependent on single-source suppliers in high-tariff jurisdictions may confront compressed gross margins or will be forced to pass cost increases onto providers and patients, which could dampen adoption in price-sensitive segments. Importantly, tariff-driven cost pressure may accelerate moves toward modularization and component substitution where clinically acceptable, enabling partial localization of supply and reducing reliance on tariff-prone imports.
In parallel, commercial teams should anticipate payer scrutiny on any price adjustments and proactively demonstrate value through adherence improvement, reduced downstream costs, and measurable clinical outcomes. Scenario planning that models supply chain alternatives, tariff pass-through thresholds, and manufacturing relocation timelines will be essential to sustain commercial competitiveness under evolving trade regimes.
Deep segmentation-driven insights revealing how device types, component choices, modes of operation, end-user settings, and distribution channels must align to maximize clinical adoption and commercial returns
Segmentation nuances drive distinct product development and commercial imperatives across device types, components, modes of operation, end users, and distribution channels. Device type considerations span Automatic Positive Airway Pressure, Bilevel Positive Airway Pressure, and Continuous Positive Airway Pressure, with bilevel platforms subdivided into Spontaneous Mode, Spontaneous Timed Mode, and Timed Mode capabilities while continuous platforms are further differentiated by Auto Adjusting and Fixed Pressure variants; these distinctions guide algorithm complexity, user interface design, and clinical positioning.Component-level segmentation-Accessories, Humidifier, Machine, and Mask-creates bundling and aftermarket opportunities; filters and tubing within accessories, integrated and standalone humidification choices, and mask options including Full Face, Nasal, and Nasal Pillow designs each influence patient comfort, adherence trajectories, and replacement cadence. Mode-of-operation segmentation across Auto Adjusting, Dual Level, and Fixed Pressure systems, with the Dual Level category further differentiated by Spontaneous, Spontaneous Timed, and Timed modes, shapes the clinical use cases prioritized by respiratory specialists and sleep clinics.
End-user variability across Home Care, Hospitals, and Sleep Clinics, with home care further segmented into Nursing Care Facility and Patient Residence and hospitals divided into General Hospital and Specialty Clinic settings, requires tailored packaging, service models, and training protocols. Distribution channel distinctions between Offline and Online, with offline channels including Hospital Suppliers, Retail Pharmacies, and Specialty Stores and online channels comprising Company Websites and E-Commerce Portals, determine margin structures, promotional tactics, and customer experience design. Organizations that align product specifications, service propositions, and go-to-market approaches to these layered segments will capture superior clinical and commercial outcomes.
Actionable regional intelligence that explains how regulatory diversity, reimbursement paradigms, and care delivery maturity drive differentiated strategies across global markets
Regional dynamics shape competitive playbooks as regulatory regimes, reimbursement philosophies, and care delivery models diverge. The Americas exhibit mature reimbursement frameworks and established home care infrastructures, which favor advanced feature sets, integrated remote monitoring, and value-based contracting arrangements. In this region, manufacturers and channel partners must emphasize clinical validation, long-term adherence data, and outcomes-based value propositions to secure placements within provider networks and payer formularies.Europe, Middle East & Africa combines highly regulated markets with diverse health system maturities, requiring flexible product portfolios and differentiated commercial approaches. Robust regulatory pathways in parts of Europe drive a premium on compliance and post-market surveillance, while emerging markets within the region demand cost-effective, robust devices and scalable distribution partnerships. Strategic entrants should balance regulatory investment with targeted local partnerships and adaptable service models.
Asia-Pacific presents a heterogeneous landscape with rapid adoption in urban centers and growing demand in rural and peri-urban segments. Local manufacturing initiatives, favorable manufacturing economics, and expanding home care capabilities create opportunities for both innovation-led premium products and competitively priced platforms. Across regions, success depends on aligning clinical evidence generation, pricing strategies, and channel partnerships to the unique regulatory and payer environments of the Americas, Europe, Middle East & Africa, and Asia-Pacific.
Insightful company-level analysis illuminating how portfolio breadth, connectivity ecosystems, and service models are reshaping competitive differentiation across patient and provider channels
Competitive dynamics in the sleep apnea device landscape are defined by product portfolio breadth, data services, and the ability to deliver integrated patient experiences. Market-leading manufacturers have invested heavily in algorithm refinement, connectivity ecosystems, and mask ergonomics to reduce non-adherence, support remote titration, and create defensible service offerings. Strategic collaboration between device makers, digital health vendors, and clinical partners has become a recurring pattern as companies seek to embed their devices into broader chronic care pathways.Smaller innovators are carving niches through focused differentiation-such as ultra-quiet machines, novel humidification approaches, or specialized mask materials-that address specific patient pain points. At the same time, channel partners and distributors that offer value-added services like training, maintenance, and bundled consumable programs play an increasingly important role in procurement decisions. Regulatory compliance and post-market data transparency are important points of differentiation; companies that proactively publish real-world evidence and support interoperability stand to win longer-term trust from providers and payers.
Forward-looking firms are also exploring adjacent opportunities in sleep diagnostics, integrated therapy-monitoring platforms, and subscription models that blend hardware, consumables, and software-driven coaching to create recurring revenue streams and deeper patient engagement.
Practical and prioritized recommendations that link product modularity, outcomes measurement, distribution optimization, and supply chain resilience to sustained competitive advantage
Industry leaders should prioritize a set of coordinated actions to secure sustainable advantage as market dynamics evolve. First, invest in modular product architectures that allow component-level localization and flexible configurations across Automatic Positive Airway Pressure, Bilevel Positive Airway Pressure, and Continuous Positive Airway Pressure platforms; this reduces tariff exposure and accelerates responses to clinical feedback. Second, institutionalize data-driven outcome measurement by integrating remote monitoring, adherence analytics, and evidence generation into product lifecycles to support payer negotiations and provider adoption.Third, optimize distribution by balancing direct online engagement with strengthened offline relationships among hospital suppliers, retail pharmacies, and specialty stores, ensuring consistent service standards and reliable consumable replenishment. Fourth, build supply chain resilience through multi-sourcing strategies, strategic nearshoring of critical assemblies, and design for manufacturability that reduces exposure to single-supplier disruption. Fifth, implement targeted pricing strategies and commercial pilots in heterogeneous regional markets to validate value propositions and inform scalable rollouts.
Collectively, these actions-centered on modular design, outcomes transparency, channel optimization, supply chain resilience, and regionally calibrated commercialization-will equip organizations to navigate tariff uncertainty, regulatory evolution, and shifting care models while driving adoption and long-term engagement.
A transparent, multi-layered research methodology combining clinician interviews, technical evaluation, regulatory review, and data triangulation to ensure actionable and reproducible insights
The research approach combines multi-source evidence gathering, expert validation, and iterative synthesis to ensure robustness and reproducibility. Primary research components include structured interviews with clinicians, procurement leaders, and channel executives to capture decision criteria, adoption barriers, and real-world performance expectations. These qualitative inputs are complemented by device-level technical reviews and usability assessments that evaluate algorithm behavior, humidification strategies, and mask interface design under representative clinical conditions.Secondary research encompasses regulatory documentation, clinical guideline analysis, and reviewed public filings to construct a comprehensive view of compliance requirements and product positioning. Data triangulation techniques reconcile divergent inputs and highlight consensus trends versus outlier observations, while sensitivity analysis explores the operational implications of policy shifts such as tariffs and reimbursement adjustments. Methodological rigor is maintained through transparent documentation of inclusion criteria, interview protocols, and evidence weighting, enabling replication and follow-up investigation.
Quality assurance includes peer review by independent clinical advisors and cross-functional validation by experts in supply chain, commercial strategy, and medical affairs. This layered methodology ensures that findings are actionable, defensible, and aligned with the practical needs of manufacturers, providers, and channel partners.
A concise conclusion synthesizing the imperative for innovation, evidence generation, and operational resilience to navigate an evolving sleep apnea device environment
In summary, the sleep apnea device sector is undergoing a period of substantive change driven by technological refinement, shifting care delivery models, and evolving trade considerations. These forces are prompting manufacturers and channel partners to rethink product architectures, accelerate digital integration, and fortify supply chains. Success will derive from the ability to align device capabilities with measurable clinical outcomes, to deploy flexible distribution strategies across both online and offline channels, and to adapt manufacturing footprints in response to policy and tariff shifts.Stakeholders that prioritize modular design, robust post-market evidence, and patient-centric interfaces will reduce adherence barriers and improve clinical effectiveness. At the same time, proactive scenario planning around tariffs and sourcing will mitigate margin pressure and enable competitive pricing strategies in diverse regional contexts. The path forward demands a balanced approach that combines innovation with operational discipline, continuous evidence generation, and collaborative partnerships across clinical, regulatory, and commercial domains.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Sleep Apnea Devices Market
Companies Mentioned
The key companies profiled in this Sleep Apnea Devices market report include:- 3B Medical, Inc.
- Apex Medical Corp.
- BMC Medical Co., Ltd.
- Braebon Medical Corporation
- Cadwell Laboratories Inc.
- Circadiance LLC
- Compumedics Limited
- DeVilbiss Healthcare GmbH
- Drive DeVilbiss Healthcare (Drive Medical)
- Fisher & Paykel Healthcare Corporation Limited
- Inspire Medical Systems, Inc.
- Koninklijke Philips N.V.
- Löwenstein Medical Technik GmbH & Co. KG
- Natus Medical Incorporated
- Philips Healthcare
- ResMed Inc.
- Somnetics International, Inc.
- SunMed, LLC.
- Weinmann Gebrauchsgüter GmbH & Co. KG
- ZOLL Respicardia, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
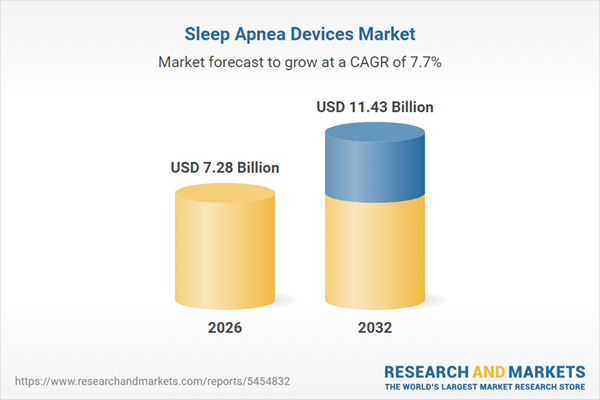
| Estimated Market Value ( USD | $ 7.28 Billion |
| Forecasted Market Value ( USD | $ 11.43 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |