The U.S. government has started various programs to raise awareness about reproductive & sexual health, especially among teenagers & young women, and provides grants to prevent unplanned pregnancies. For example, the Office of Population Affairs Teen Pregnancy Prevention (TPP) Program provides funding to diverse organizations working to prevent teen pregnancies across the U.S. Such proactive steps are expected to increase the adoption of LARCs among women, thus, favoring the market growth.
LARCs are highly effective compared to short-term contraceptive methods. They are suitable for teenagers as they are less likely to maintain regular dosage or implement other short-term contraceptives. Hence, the government is implementing various programs for the free distribution of Intrauterine Devices (IUDs) among adolescents. Furthermore, according to the Kaiser Family Foundation, in 2015-2017, the use of subdermal implants was highest among teens (aged 15 to 19 years), with 16% of them using implants. This, coupled with increasing funding by the government on family planning is anticipated to drive the market.
The COVID-19 pandemic has had a negative impact on the contraceptives market, and its magnitude on the LARC has been even more. Unlike other contraceptives, such as condoms and oral pills, the use of LARC requires visiting a medical facility for the insertion of an IUD or implant. Hence, with the postponement of elective procedures and people avoiding hospital visits, the sales of long-acting contraceptives declined. For instance, the new placement of PARAGRAD dropped by about 65% in April and 40% in May in 2020 compared to the same period in 2019.
U.S. Long-acting Contraception Market Report Highlights
- The United States has the highest rate of adolescent pregnancies. Government programs, such as improving sex education and increasing contraception availability, are projected to increase the use of various birth control techniques including LARC in the country
- In 2020, the IUD segment held the maximum market share owing to the easy availability and convenience of use. The segment is expected to grow at the highest CAGR from 2021 to 2028 owing to the increasing demand for hormonal IUDs
- Key companies in this market are focusing more on distribution agreements, supply contracts, and product development to gain a competitive edge over others. Furthermore, many companies, such as Allergan, are partnering with organizations for free distribution of their products to strengthen their market position
- Increasing research by potential new entrants, such as Cipla, Inc. and Sebela Pharmaceuticals, Inc., is anticipated to change the competitive landscape of the market in the coming years
- For example, in January 2019, Cipla Inc. received ANDA approval for the generic version of Medroxyprogesterone Injectable, 150mg/mL from the U.S. FDA
Table of Contents
Chapter 1 Methodology and Scope1.1 Market Segmentation & Scope
1.1.1 Estimates and Forecast Timeline
1.2 Research Methodology
1.3 Information Procurement
1.3.1 Purchased Database
1.3.2 Internal Database
1.3.3 Secondary Sources
1.3.4 Primary Research
1.3.5 Details Of Primary Research
1.4 Information Or Data Analysis
1.4.1 Data Analysis Models
1.5 Market Formulation & Validation
1.6 Model Details
1.6.1 Commodity Flow Analysis
1.7 List Of Secondary Sources
1.8 List Of Abbreviations
1.9 Objectives
1.9.1 Objective 1
1.9.2 Objective 2
1.9.3 Objective 3
Chapter 2 Executive Summary
2.1 Market Outlook
2.2 Segment Outlook
2.2.1 Product
2.3 Competitive Insights
Chapter 3 Market Variables, Trends, & Scope
3.1 Market Lineage Outlook
3.1.1 Parent Market Outlook
3.1.2 Related/Ancillary Market Outlook
3.2 Market Dynamics
3.2.1 Market Driver Analysis
3.2.1.1 Increasing Adoption Of Long-Acting Contraceptives
3.2.1.2 Strong Government Support
3.2.1.3 Rising Publicly Funded Family Planning Services
3.2.2 Market Restraint Analysis
3.2.2.1 Less Approved Products
3.2.2.2 Adverse Side Effects Associated With Use Of Larc
3.3 Business Environment Analysis Tools
3.3.1 Pestle Analysis
3.3.1.1 Political & Legal Landscape
3.3.1.2 Economic & Social Landscape
3.3.1.3 Technological Landscape
3.3.2 Porter’s Five Forces Analysis
3.3.2.1 Competitive Rivalry: Moderate
3.3.2.2 Bargaining Power Of Suppliers: Moderate
3.3.2.3 Bargaining Power Of Buyers: High
3.3.2.4 Threat Of New Entrants: High
3.3.2.5 Threat Of Substitutes: Moderate
3.4 Penetration & Growth Prospect Mapping
3.5 Regulatory Framework and Reimbursement Analysis
3.5.1 Regulatory Scenario
3.5.2 Reimbursement Framework
3.6 Impact of COVID-19: Qualitative Analysis
3.7 Demographic Analysis: Methods of Contraception, 2019
Chapter 4 U.S. Long-acting Contraception Market: Product Analysis
4.1 U.S. Long-acting Contraception Market Share analysis, 2020 & 2028
4.2 Segment Dashboard
4.3 Market Size & Forecasts and Trend Analysis, 2016 to 2028 for the Product
4.3.1 Intrauterine Devices (IUD)
4.3.1.1 Intrauterine Devices (IUD) market, 2016 - 2028 (USD Million)
4.3.1.2 Hormonal IUD
4.3.1.2.1 Hormonal IUD market, 2016 - 2028 (USD Million)
4.3.1.3 Nonhormonal IUD
4.3.1.3.1 Nonhormonal IUD market, 2016 - 2028 (USD Million)
4.3.2 Subdermal Implants
4.3.2.1 Subdermal Implants market, 2016 - 2028 (USD Million)
4.3.3 InjecTable
4.3.3.1 InjecTable market, 2016 - 2028 (USD Million)
Chapter 5 Competitive Analysis
5.1 Recent Developments & Impact Analysis, by Key Market Participants
5.2 Competition Categorization
5.3 Company Market Position Analysis
5.4 Company Profiles
5.4.1 MERCK & CO., INC.
5.4.1.1 Company Overview
5.4.1.2 Financial performance
5.4.1.3 Product Benchmarking
5.4.1.4 Strategic Initiatives
5.4.1.5 SWOT analysis
5.4.2 PFIZER INC.
5.4.2.1 Company Overview
5.4.2.2 Financial performance
5.4.2.3 Product Benchmarking
5.4.2.4 SWOT analysis
5.4.3 THE COOPER COMPANIES INC.
5.4.3.1 Company Overview
5.4.3.2 Financial performance
5.4.3.3 Product Benchmarking
5.4.3.4 Strategic Initiatives
5.4.3.5 SWOT analysis
5.4.4 ALLERGAN (ABBVIE INC.)
5.4.4.1 Company Overview
5.4.4.2 Financial performance
5.4.4.3 Product Benchmarking
5.4.4.4 Strategic Initiatives
5.4.4.5 SWOT analysis
5.4.5 BAYER AG
5.4.5.1 Company Overview
5.4.5.2 Financial performance
5.4.5.3 Product Benchmarking
5.4.5.4 Strategic Initiatives
5.4.5.5 SWOT analysis
Chapter 6 KOL Comments
List of Tables
Table 1 List of secondary sources
Table 2 List of abbreviation
Table 3 Long-acting contraceptives and associated adverse effects
Table 4 Classification of contraceptives by the U.S. FDA
Table 5 States that have expanded on contraceptive coverage guarantee in Affordable Care Act
Table 6 List of intrauterine devices currently available in the U.S.List of Figures
FIG. 1 U.S. long-acting contraception market segmentation
FIG. 2 Market research process
FIG. 3 Data triangulation techniques
FIG. 4 Primary research pattern
FIG. 5 Market research approaches
FIG. 6 Value-chain-based sizing & forecasting
FIG. 7 QFD modeling for market share assessment
FIG. 8 Market formulation & validation
FIG. 9 Market outlook, 2020 (USD Million)
FIG. 10 Market trends & outlook
FIG. 11 Market driver relevance analysis (Current & future impact)
FIG. 12 Market restraint relevance analysis (Current & future impact)
FIG. 13 Penetration & growth prospect mapping
FIG. 14 Percentage of women (aged 15-49) using modern contraceptive methods in U.S., 2019
FIG. 15 Percentage of women (aged 15-49) using traditional contraceptive methods in U.S., 2019
FIG. 16 U.S. long-acting contraception market: Product movement analysis (USD Million)
FIG. 17 U.S. long-acting contraception treatment outlook: Key takeaways (USD Million)
FIG. 18 Intrauterine devices (IUD) market, 2016 - 2028 (USD Million)
FIG. 19 Hormonal IUD market, 2016 - 2028 (USD Million)
FIG. 20 Nonhormonal IUD market, 2016 - 2028 (USD Million)
FIG. 21 Subdermal implants market, 2016 - 2028 (USD Million)
FIG. 22 InjecTable market, 2016 - 2028 (USD Million)
FIG. 23 Recent development & impact analysis by key market participants
FIG. 24 Competition categorization
FIG. 25 Company market position analysis
Companies Mentioned
- MERCK & CO. INC.
- PFIZER INC.
- THE COOPER COMPANIES INC.
- ALLERGAN (ABBVIE INC.)
- BAYER AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 76 |
| Published | December 2021 |
| Forecast Period | 2021 - 2028 |
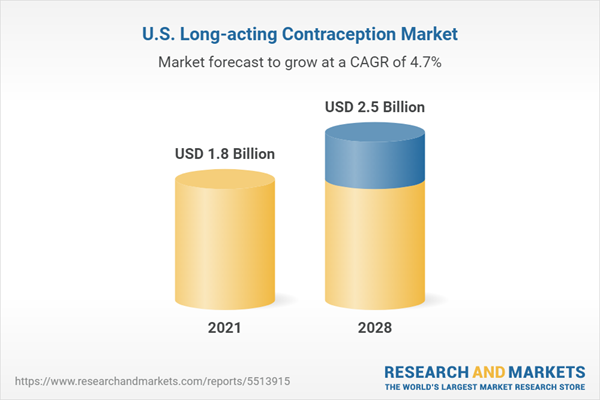
| Estimated Market Value ( USD | $ 1.8 Billion |
| Forecasted Market Value ( USD | $ 2.5 Billion |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | United States |
| No. of Companies Mentioned | 5 |