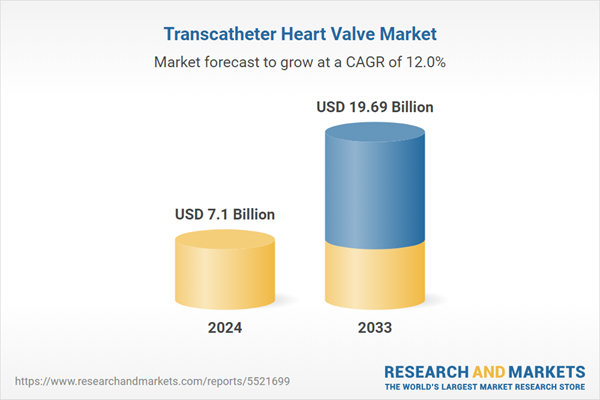
Transcatheter Heart Valve Market is expected to reach US$ 19.69 Billion in 2033 from US$ 7.10 Billion in 2024, with a CAGR of 12.00% from 2025 to 2033. Ageing populations, a growth in cardiovascular disorders, improvements in minimally invasive technology, higher procedural success rates, and patient demand for safer treatments are the main factors driving the transcatheter heart valve replacement (THVR) market.
Transcatheter Heart Valve Replacement Market Global Report by Type (Transcatheter Aortic Valve Replacement, Transcatheter Mitral Valve Replacement, Transcatheter Pulmonary Valve Replacement), Material (Mechanical valves, Biological Valves), End-User (Hospitals, Ambulatory Surgical Centers, Others), Countries and Company Analysis 2025-2033.
Transcatheter Heart Valve Replacement Industry Overview
Patients with severe heart valve disease, especially aortic stenosis, can be treated with transcatheter heart valve replacement (THVR), a minimally invasive treatment. A catheter is guided to the heart during the surgery by being implanted into a blood artery, usually in the groin. The damaged valve is then replaced without open heart surgery by implanting a new, bioprosthetic valve. For high-risk patients or those unable to have standard surgery, THVR is the best option. The treatment is revolutionary in cardiovascular care since it shortens recovery times, decreases problems, and improves results when compared to standard valve replacement.
A number of factors are propelling the Transcatheter Heart Valve Replacement (THVR) market's expansion. Globally, the prevalence of cardiovascular illnesses, especially aortic stenosis, is rising as people age. High-risk patients who are unable to have open heart surgery can now choose THVR thanks to advancements in minimally invasive technologies and increased procedural success rates. Demand is also fueled by growing awareness of the advantages of THVR, such as shortened recovery times and fewer problems. Additionally, the market is developing as a result of healthcare professionals' increasing use of THVR procedures and the expansion of healthcare access. Market expansion is still being driven by ongoing research and product innovation.
Growth Drivers for the Transcatheter Heart Valve Replacement Market
Minimally Invasive Procedures
One of the main factors propelling the market for transcatheter heart valve replacement (THVR) is minimally invasive methods. These methods provide patients with a less traumatic and safer option than open heart surgery. With THVR, a new valve is inserted via a catheter, frequently through a little incision. This procedure speeds up hospital discharge, lowers the risk of complications, and greatly shortens recovery times. The use of THVR is growing as more patients choose less invasive procedures that have lower risks and a faster recovery. The market is growing as a result of this trend and developments in catheter-based technologies, which are increasing the use of THVR in the treatment of cardiovascular disorders.
Rising geriatric population
among the main factors propelling the market for transcatheter heart valve replacement (THVR) is the aging population. Age-related cardiovascular diseases, such as aortic stenosis, are becoming more common as the world's senior population grows.One in six individuals worldwide will be 60 years of age or older by 2030. At this point, there will be 1.4 billion people over the age of 60, up from 1 billion in 2020. The number of individuals in the world who are 60 years of age or older is expected to increase to 2.1 billion by 2050.Heart valve replacement treatments are in greater demand as a result of this demographic transition, especially for high-risk elderly patients who might not be good candidates for open heart surgery. Consequently, THVR turns into a necessary, non-intrusive choice that speeds up the market.
Ongoing Research & Development
The market for transcatheter heart valve replacement (THVR) is growing significantly due in large part to ongoing research and development (R&D). In addition to increasing the number of patients eligible for THVR, ongoing developments in valve design, materials, and delivery methods are also improving procedural outcomes. Innovations improve performance and lower problems, such as the creation of valves that are more patient-specific, flexible, and long-lasting. Additionally, R&D efforts are concentrated on improving imaging technologies to guarantee better clinical outcomes and more precise device installation. THVR keeps gaining traction as new technologies and procedural approaches develop, propelling market expansion and enhancing patient results.
In Nov 2024, at significant cardiology conferences, Meril Life Sciences showcased its Myval Octapro Transcatheter Heart Valve (THV), showcasing its technological breakthroughs in valve replacement. The novel valve is intended to enhance patient compatibility and procedure results. Meril's commitment to improving treatment for structural heart disease is demonstrated by its continuous research and innovation.
Challenges in the Transcatheter Heart Valve Replacement Market
High costs
One major obstacle in the market for transcatheter heart valve replacements (THVRs) is high pricing. The technique requires highly skilled medical personnel, costly gadgets, and specialized equipment, which raises the total cost of therapy. This may restrict patient access, particularly in areas with lower incomes or in healthcare systems with tighter finances. Even while THVR has advantages including a quicker recovery time, its expensive cost prevents it from being widely used, so finding affordable alternatives is crucial for future market expansion.
Existence of Substitute Products to Complicate the Market
Despite the benefits of minimally invasive transcatheter technology, traditional surgical valves are still used in many places, particularly in developing nations. Patients have a considerable need for the surgical valves. This is mostly due to the fact that surgical valves can last a lifetime and are more robust than transcatheter ones. One of the main things that can hurt the industry is the well-established existence of surgical valves.
Transcatheter Heart Valve Replacement Market Overview by Regions
Regional growth trends are evident in the market for transcatheter heart valve replacement (THVR). North America is in the lead because of its aging population, strong adoption rates, and sophisticated healthcare infrastructure. Demand is high in Europe, especially in nations like Germany and the UK. The Asia Pacific region is expanding quickly as a result of increased incidence of cardiovascular disease and better access to healthcare. Growing healthcare investments are fueling the market's slow expansion in Latin America and the Middle East.
United States Transcatheter Heart Valve Replacement Market
Due to an aging population and a high prevalence of cardiovascular illnesses, especially aortic stenosis, the United States Transcatheter Heart Valve Replacement (THVR) market is one of the largest segments in the world. The broad adoption of THVR is made possible by the nation's sophisticated healthcare infrastructure, easy access to state-of-the-art medical technology, and robust reimbursement support. Particularly for high-risk patients, THVR operations are becoming more and more popular over traditional surgery due to established clinical criteria and skilled specialists. The United States is positioned as a major player in the worldwide THVR market thanks to continued research, innovative valve design, and growing healthcare access.
In March 2024, for patients with symptomatic severe aortic stenosis, Medtronic announced that the FDA has authorized its Evolut FX+ transcatheter aortic valve replacement (TAVR) system. The diamond-shaped frame of this upgraded system provides improved coronary access while preserving the excellent valve performance for which the Evolut platform is renowned. Within two years, heart failure might result from severe aortic stenosis if treatment is not received.
Germany Transcatheter Heart Valve Replacement Market
Germany's sophisticated healthcare system, first-rate medical infrastructure, and aging population make it one of the continent's top markets for transcatheter heart valve replacement (THVR). The prevalence of heart valve disorders, such as aortic stenosis, raises the need for THVR operations. The extensive use of minimally invasive heart valve replacements is further aided by Germany's robust reimbursement regulations and highly qualified medical personnel. The market is also growing as a result of the nation's strong clinical research and development initiatives, with ongoing advancements in THVR technology improving patient results and increasing available treatments.
India Transcatheter Heart Valve Replacement Market
The market for transcatheter heart valve replacement (THVR) in India is expanding quickly as a result of an aging population, increased prevalence of cardiovascular illness, and better healthcare facilities. Adoption has been accelerated by advances in medical technology and growing knowledge of minimally invasive procedures. The growing middle class and government programs to increase access to healthcare are propelling market expansion, even if cost is still a barrier. The robust medical tourism industry in India is another factor driving up demand for THVR procedures.
Saudi Arabia Transcatheter Heart Valve Replacement Market
The market for transcatheter heart valve replacement (THVR) in Saudi Arabia is expanding quickly because to the country's aging population and rising prevalence of cardiovascular illnesses. The need for minimally invasive procedures like THVR is growing since cardiovascular diseases are one of the main causes of death. Government efforts to improve medical services and a strong healthcare infrastructure benefit the nation. The market is also growing as a result of the acceptance of these life-saving operations being fueled by improvements in THVR technology and growing patient awareness.
Transcatheter Heart Valve Replacement Market Segments
Type
1. Transcatheter Aortic Valve Replacement
2. Transcatheter Mitral Valve Replacement
3. Transcatheter Pulmonary Valve Replacement
Material
1. Mechanical valves
2. Biological Valves
End-User
1. Hospitals
2. Ambulatory Surgical Centers
3. Others
Countries
North America
1. United States
2. Canada
Europe
1. France
2. Germany
3. Italy
4. Spain
5. United Kingdom
6. Belgium
7. Netherlands
8. Turkey
Asia Pacific
1. China
2. Japan
3. India
4. South Korea
5. Thailand
6. Malaysia
7. Indonesia
8. Australia
9. New Zealand
Latin America
1. Brazil
2. Mexico
3. Argentina
Middle East & Africa
1. Saudi Arabia
2. UAE
3. South Africa
Rest of the World
All the Key players have been covered from 5 Viewpoints:
1. Overview
2. Key Persons
3. Product Portfolio
4. Recent Development & Strategies
5. Sales Analysis
Key Players Analysis
1. Edwards Lifesciences
2. Abbott Laboratories
3. Medtronic Plc
4. LIVANOVA PLC
5. Boston Scientific Corporation
6. Artivion, Inc.
7. MicroPort Scientific Corporation
8. Venus Medtech (Hangzhou) Inc.
Table of Contents
Companies Mentioned
- Edwards Lifesciences
- Abbott Laboratories
- Medtronic Plc
- LIVANOVA PLC
- Boston Scientific Corporation
- Artivion, Inc.
- MicroPort Scientific Corporation
- Venus Medtech (Hangzhou) Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 7.1 Billion |
| Forecasted Market Value ( USD | $ 19.69 Billion |
| Compound Annual Growth Rate | 12.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |