COVID-19 had a significant impact on the growth of the market during the pandemic period. The increasing amount of medical waste generated during the COVID-19 outbreak has become a considerable concern worldwide. For instance, according to the data published by WHO in February 2022, tonnes of extra medical waste from the response to the COVID-19 pandemic has put tremendous strain on healthcare waste management systems worldwide. Thus, to overcome such circumstances, the demand for single-use medical device reprocessing increased, contributing to the market's growth during the pandemic. In addition, to maintain sustainable waste management systems even during the post-pandemic situation, the demand for single-use medical device reprocessing is expected to be on the rise, thereby boosting the growth of the studied market over the coming five years.
Rising clinical urgency to minimize medical waste generation in healthcare settings and hospitals is among the key factors driving the market growth. As per the BMC article published in April 2020, Brazil Permits reprocessing of single-use medical devices used for many intervention procedures. The article also mentioned that the lack of essential guidance on how to carry out the practice is urgent in need. Such favorable government permits and the need for guidance are expected to drive the demand for single-use medical device reprocessing, thereby contributing to market growth over the forecast period.
The use of reprocessed single-use medicals also reduces costs associated with special handling and waste management of devices. Healthcare facilities save 50% for every reprocessed Single-use medical device they purchase and spend less on medical disposal. They also save money when the original equipment manufacturers (OEMs) lower their prices to compete with third-party reprocessors, thus reducing the overall cost of healthcare.
Along with cost reduction, it also results in environmental sustainability. Single-use medical device reprocessing is a top healthcare supply chain strategy to reduce costs and optimize resources. The factors above are anticipated to contribute towards industrial development in the immediate future. However, the lack of dedicated Single-use device reprocessing facilities in South American markets is currently a massive opportunity for this market. In contrast, the unauthorized overuse of reprocessed Single-use medical devices in emerging markets is currently the major challenge faced by this market.
South America Single-Use Medical Device Reprocessing Market Trends
Sequential Compression Sleeves by Class II Device Segment is Poised to Register Robust Growth
The class II device segment is expected to hold a significant share in the single-use medical device reprocessing market over the forecast period, owing to the increasing number of cardiac and other intervention procedures and the consequent economic load demand for assessing single-use device reuse due to the rising cost of instruments and burden of cardiovascular diseases around the world. In class II, sequential compression sleeves are designed to increase venous blood flow in patients with deep vein thrombosis and pulmonary embolism. Compression sleeves are considered reusable, as they can withstand cleaning and sterilization. The quality of the device and physical characteristics are not likely to be affected by reprocessing, and the device remains safe and effective for its intended use.Sequential compression sleeves are used widely worldwide due to the high burden of diseases such as deep vein thrombosis and pulmonary embolism. They are further expected to increase over the years. As per the NCBI article published in January 2020, the incidence rate of venous thromboembolism in Argentina was 0.7 per 1,000 person-years. Furthermore, with the growing burden of cardiovascular diseases, sequential compression sleeves are expected to increase, which will drive growth in the studied segment. Hence, the sequential compression sleeve segment is expected to grow due to the rising medical device demand.
Brazil is Expected to Dominate the Single-use Medical Device Reprocessing Market over the Forecast Period
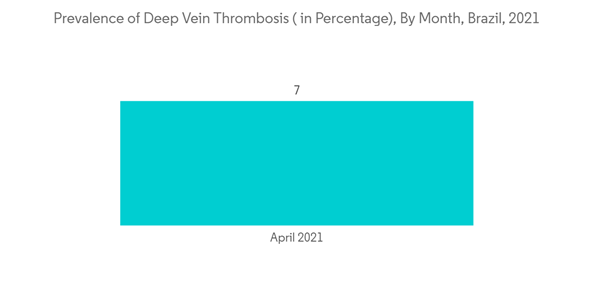
Brazil holds a major share in the single-use medical device reprocessing market and is expected to show a similar trend over the forecast period, mainly due to the increasing cost of reusable medical devices and the rising prevalence of cardiovascular diseases in the region.The increase in the demand for single-use medical device reprocessing in Brazil is due to the high burden of diseases such as deep vein thrombosis and pulmonary embolism, which are further expected to increase over the years. As per the NIH article published in February 2022, the average prevalence of deep vein thrombosis was 2.5% among the Brazillian population. The months of March and April 2021 had a significant increase in venous thrombosis and mortality. Also, increasing cases of cardiovascular diseases and related surgical procedures are the major growth factors for the market. As per the NCBI article published in June 2020, cardiovascular diseases were responsible for more than 10.0% of all hospital admissions in Brazil. Similarly, in another article published in NCBI in September 2021, the average annual rate for coronary heart disease and heart stroke was 78.75 among the Brazillian population.
Thus, the prevalence of cardiac diseases generates a growing need for single-use medical devices and reprocessing, and this high prevalence of diseases requiring class I and class II devices will significantly boost the market's growth in this country.
South America Single-Use Medical Device Reprocessing Market Competitor Analysis
The South America Single-use Medical Device Reprocessing Market is moderately competitive and consists of several major players. A few significant players currently dominate the market in terms of market share. The major market players include Hygia, Medline Renewal, NEScientific, ReNu Medical, and SterilMed.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Hygia
- Medline Industries Inc.
- NEScientific
- Arjo (ReNu Medical)
- Johnson & Johnson (Sterilmed Inc.)
- Stryker Corporation
- SureTek Medical
- Vanguard