COVID-19 had a profound impact on the rare neurological disease treatment market owing to the cancellations in elective procedures, including neurological treatment and diagnosis, which impacted the market. The clinical trials were also suspended due to the COVID-19 restrictions, which delayed the drug review and approval process. Some research articles reported the high prevalence of rare neurological diseases after taking COVID-19 vaccines. For instance, as per the study published in June 2021 in Nature Journal, the frequency of Guillain-Barre syndrome in India and England was estimated to be ten times greater than expected after taking the COVID-19 vaccine. However, the market started recovering since the restrictions were lifted and rare neurological disease treatments resumed.
In addition, the increasing prevalence of rare neurological diseases, promising pipeline drugs for the treatment of rare neurological disorders, and favorable government policies worldwide for speeding up diagnostic processes are actively affecting the growth of the studied market.
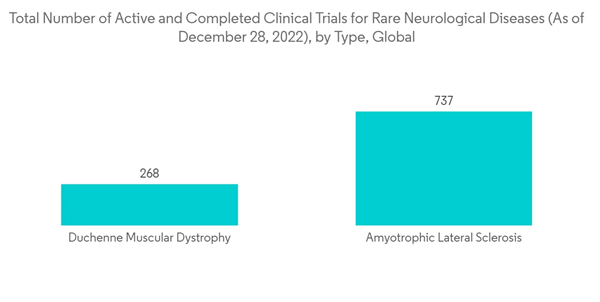
Some rare neurological diseases include amyotrophic lateral sclerosis (ALS), Duchenne muscular dystrophy, Vertical gaze palsy, and Huntington's disease, among others. As per the Orphanet report published in January 2022, the prevalence of chronic inflammatory demyelinating polyneuropathy is 3.7 per 100,000 individuals in Europe, and the prevalence of ALS is 3.85 worldwide and 5.2 in European Union (EU) in 2021. As per the same source, the prevalence of Huntington's disease is 12.0 in the EU in 2021. The high prevalence of rare neurological diseases is projected to boost the demand for their treatment, which is estimated to propel the launch of more drugs, and ultimately augment the market growth in the forecast period.
Furthermore, the strategic initiatives by key players, such as product launches, product approval, research studies, and partnerships, are expected to propel the market growth. For instance, in August 2021, Novartis announced the lift of a partial clinical trial hold and initiated a new, pivotal Phase 3 study of intrathecal OAV-101 in older patients with spinal muscular atrophy. Also, in January 2022, Novartis announced that the U.S. Court of Appeals for the Federal Circuit (CAFC) issued its decision upholding the validity of US Patent No. 9,187,405, covering a dosing regimen for Gilenya. The rising clinical trial activities and launches of drugs for rare neurological disease treatment are expected to propel the market growth during the forecast period.
Therefore, owing to the factors such as the rising burden of rare neurological diseases and demand for treatment of the diseases, rising clinical trial studies to develop a new intervention are expected to augment the market growth over the analysis period. However, the high cost of rare neurological disease treatment is expected to hinder the growth of the rare neurological disease treatment market.
Rare Neurological Disease Treatment Market Trends
The Small Molecules Segment is Expected to Hold a Major Share in the Rare Neurological Disease Treatment Market
A small molecule is a drug that can enter cells quickly due to its low molecular weight. The segment holds a significant share of the rare neurological disease treatment market and is anticipated to show a similar trend over the forecast period due to the higher cost of biological drugs.According to a research article published in August 2021 in Neurotherapeutics Journal, small molecules can be used to treat genetic epilepsies and rare or ultrarare genetic epilepsy. The advantage of the small molecules is their size, which allows access to extracellular and intracellular targets for modulation of discrete protein functions, such as ion channel gating. These substances can also be made more quickly, in large numbers, and at an economical cost, making them useful for developing medicines for rare disorders in the upcoming years. Such research studies are likely to create opportunities and increase the demand for small molecules to develop rare neurological diseases.
Furthermore, as per an article published Orphanet Journal of Rare Diseases in March 2021, the survey on research priorities was designed by the ERN for Rare Neurological Diseases (ERN-RND) working group on research and registries. In this, both patient representatives and healthcare professionals were asked to prioritize five research themes for rare neurological diseases, as patient involvement in research increases the impact of research and the likelihood of adoption in clinical practice. Such studies will raise awareness regarding rare diseases, leading to high demand for small-molecule treatment options.
Moreover, with the help of collaborations among market players, the segment’s growth is mainly contributed to the research and discovery of small molecules for rare neurological diseases. For instance, in July 2021, Servier and Nymirum entered a strategic collaboration to identify and develop RNA-modulatory drugs to treat neurological diseases. Under the collaboration agreement, Nymirum will leverage its proprietary DART Platform (Dynamic Atomic-Resolution RNA Targeting Platform) to discover novel small molecule therapeutics for multiple neurological targets.
Hence, increasing incidences of rare neurological diseases worldwide, increasing focus on fast-track approvals, technological advancements, and growing awareness regarding early diagnosis of rare neurological diseases are the key factors driving the small molecules segment.
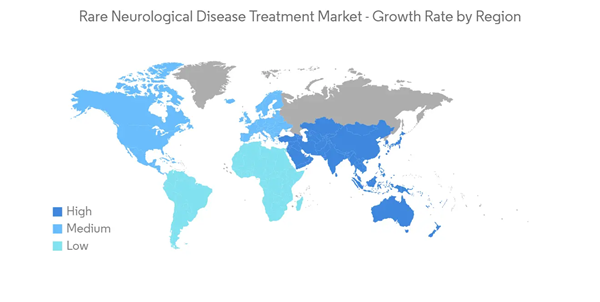
North America is Expected to Hold a Significant Market Share Over The Forecast Period
North America is expected to hold a significant share of the rare neurological disease treatment market due to the availability of reimbursement for the treatment of rare diseases, the growing incidence of rare neurological disorders, and increasing research and development (R&D) in the region.As per the Orphanet report published in January 2022, according to a StatPearls article updated in March 2022, approximately 350 cases of Creutzfeldt-Jakob disease (CJD) are diagnosed annually in the United States. Sporadic CJD is the commonest form of human prion disease, and the mean age of onset is 61 years. Thus the high prevalence in the region is expected to propel the demand for the rare neurological disease treatment market during the forecast period.
In addition, the strategic initiatives by market players, such as product launches, approvals, collaborations, and partnerships, contribute to the studied market's growth. For instance, in December 2021, Novartis received approval from the US FDA for Fast Track designation for branaplam (LMI070) to treat Huntington's disease (HD). The product launches and approvals are expected to propel the market in the region during the forecast period.
Moreover, increasing healthcare spending on R&D and the presence of well-established healthcare infrastructure are fueling the growth of the overall regional market to a large extent. For instance, as per the National Institutes of Health (NIH) RePORT 2022 update, the research spending for Huntington's disease was USD 49 million in 2020 and USD 46 million in 2021. The estimated R&D spending on Huntington's disease is USD 48 million in 2022 in the United States. The high expenditure on rare neurological disease is expected to augment the market growth in the region during the forecast period.
Rare Neurological Disease Treatment Market Competitor Analysis
The Rare Neurological Disease Treatment Market is moderately consolidated. The market consists of a few significant players that dominate the market, including CSL Ltd, Kedrion Biopharma Inc., US WorldMeds LLC (Solstice Neurosciences LLC), Merz Pharma GmbH & Co. KGaA, Aquestive Therapeutics Inc., Bayer AG, Pfizer Inc., Novartis AG, Merck & Co. Inc. (EMD Serono Inc.), Jazz Pharmaceuticals PLC, F. Hoffmann - La Roche Ltd Biogen Inc, and Teva Pharmaceutical Industries Ltd.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- US WorldMeds LLC (Solstice Neurosciences LLC)
- CSL Ltd
- Merz Pharma GmbH & Co. KGaA
- Aquestive Therapeutics Inc.
- Kedrion Biopharma Inc.
- Bayer AG
- Pfizer Inc.
- Novartis AG
- Teva Pharmaceutical Industries Ltd.
- Biogen Inc.
- F. Hoffmann - La Roche Ltd
- Merck & Co. Inc. (EMD Serono Inc.)