Key Highlights
- The COVID-19 outbreak had an unprecedented impact on the growth of the market. This outbreak resulted in the postponement and cancellation of various cardiac surgeries, affecting the demand for left atrial appendage devices. For instance, a research article published by Europe PMC in July 2021 reported a 73% decrease in cardiac procedures performed in India between April and August 2020. Such reduction in cardiac procedures had a slight negative impact on the market's growth. However, with the relaxation of the lockdown, these postponed surgeries were carried out.
- Additionally, COVID-19 has also impacted people's cardiac health, increasing the demand for left atrial appendage devices. Thus, owing to these factors, the studied market is expected to continue its growth over the coming five years.
- The key contributors to the market growth are the increasing geriatric population and associated cardiovascular diseases. Also, the rise in atrial fibrillation among the global population is expected to contribute to the market's growth.
- For example, according to the National Center for Biotechnology Information (NCBI), in February 2021, the worldwide prevalence of atrial fibrillation was 37,574 million cases (0.51% of the world's population), which also increased by 33% during the last 20 years. Such an increase in atrial fibrillation conditions among the global population is expected to drive the growth of the studied market during the forecast period.
- Moreover, people aged 65 years and above are at a higher threat of causing cardiovascular diseases such as atrial fibrillation, which might further lead to heart stroke. According to the UN Department of Economic and Social Affairs (UDESA) report, 2022, the share of the global population aged 65 years or above is projected to rise from 10% in 2022 to 16% in 2050. Thus, the increase in the global geriatric population prone to cardiovascular diseases is expected to drive the market's growth during the forecast period.
- Furthermore, increasing product developments are expected to contribute to the growth of the studied market. For instance, in June 2022, Conformal Medical Inc. announced the enrolment of the first participants in the CONFORM pivotal trial of its CLAAS System. The first patients were enrolled at two sites in the US. The CLAAS System has been designed to seal the left atrial appendage (LAA) in non-valvular atrial fibrillation (Afib) patients to reduce stroke risk without using anticoagulants. Conformal Medical noted that the findings from the investigational device exemption (IDE) study will help the company seek pre-market approval for the device from the US Food and Drug Administration (FDA).
- Therefore, the increasing prevalence of atrial fibrillation and innovative product developments are expected to contribute to the market's growth. However, the high cost of devices may restrain the growth of the market during the forecast period.
Left Atrial Appendage Devices Market Trends
Hospital Segment is Expected to Hold a Significant Share in the Left Atrial Appendage Devices Market Over the Forecast Period
- The hospital segment of the left atrial appendage devices market is expected to show significant growth. The increasing number of hospitals and advancing infrastructure, coupled with the increasing incidence of cardiovascular disorders such as atrial fibrillation, are expected to drive the demand for left atrial appendage devices, thereby contributing to the growth of the studied segment.
- Furthermore, the increasing burden of atrial fibrillation is expected to be a significant contributor to this segment. According to the NCBI, atrial fibrillation is the most frequent cardiac arrhythmia. As the population ages globally, atrial fibrillation (AF) is predicted to affect 6-12 million people in the USA by 2050 and 17.9 million in Europe by 2060. Also, another NCBI article published in May 2021 mentioned that age and gender-standardized prevalence of atrial fibrillation was highest in North America, Europe, China, and South East Asia.
- The report also mentioned that compared with low-income countries, atrial fibrillation was 7-fold higher in middle-income countries and 11-fold higher in high-income countries. Such a high prevalence of atrial fibrillation and various cardiovascular disorders is also expected to drive the growth of the studied segment over the forecast period.
- Additionally, the increasing number of hospitals is also expected to contribute to the growth of the studied segment. For instance, the American Hospital Association (AHA) statistics published in 2022 show that the total number of active hospitals in the United States was 6,093 in 2022, with 33,356,853 hospital admissions. Thus, the high volume of hospitals drives increased demand for left atrial appendage devices, thereby boosting the studied segment growth.
- Hence, factors such as the increasing number of hospitals and increasing demand for advanced left atrial appendage devices are expected to increase the demand for left atrial appendage devices in this segment and thus drive the market segment's growth.
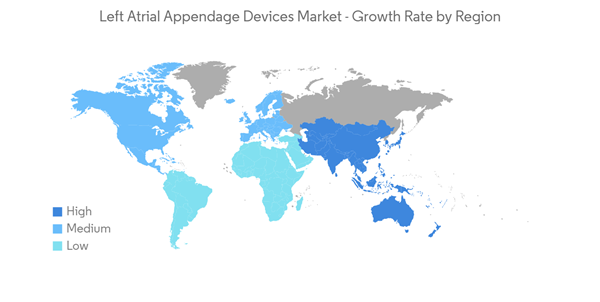
North America is Expected to Hold a Significant Share in the Market and Expected to do Same Over the Forecast Period
- North America is expected to be a dominant region in the left atrial appendage devices market owing to the growing number of cases of atrial fibrillation. Additionally, the launch of advanced products with the presence of major market players and quick adoption of the technologies are also expected to contribute to the market's growth.
- According to Centre for Disease Control and Prevention (CDC) data, October 2022, it is predicted that 12.1 million people in the United States will have atrial fibrillation by the year 2030. According to a similar NCBI analysis released in July 2021, atrial fibrillation was discovered in 498 of the 8,686 patients in the Mexican population, with a higher prevalence in men and older women. Also, with the rising geriatric population, the demand for the studied market is expected to grow in the future. Atrial fibrillation is likely to cause heart stroke, so left atrial appendage management is of utmost importance. Furthermore, novel technologies are being introduced in the market, increasing the adoption of these devices.
- Additionally, the rising geriatric population is expected to drive the market's growth in North American countries. For instance, the data published by Statistics Canada in August 2022 indicated that the population aged 70 to 74 years in Canada was 1,847,585, whereas the population aged 80 to 84 years was 840,545. Such a rise in the geriatric population prone to developing cardiovascular diseases is expected to drive the demand for left atrial appendage devices, thereby contributing to the market's growth.
- The presence of key players in the country, technological advancements, and the availability of favorable product approvals augment the growth of the market studied in the United States. For instance, in August 2021, Abbott received approval from the FDA for its Amplatzer Amulet left atrial appendage occluder to treat the device with atrial fibrillation at risk of ischemic stroke. The device offers immediate closure of the left atrial appendage - an area where blood clots can form in people suffering from atrial fibrillation - reducing their risk of stroke and immediately eliminating the need for blood-thinning medication.
- Thus, factors such as the increasing burden of cardiovascular disorders, the growing geriatric population, the presence of major market players, and product developments are expected to contribute to the market growth in this region.
Left Atrial Appendage Devices Market Competitor Analysis
The left atrial appendage devices market is fragmented in nature due to the presence of several companies operating globally as well as regionally. Key players that are expected to be dominant in left atrial appendage devices market are Abbott, Articure, Boston Scientific, Johnson and Johnson, Lifetech Scientific, Occlutech, Cardia Inc., among othersAdditional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott
- Articure
- Boston Scientific
- Johnson & Johnson (Biosense Webster)
- Lifetech Scientific
- Occlutech
- Cardia Inc
- Aegis Medical Group
- Acutus Medical Inc
- ŌNŌCOR Llc