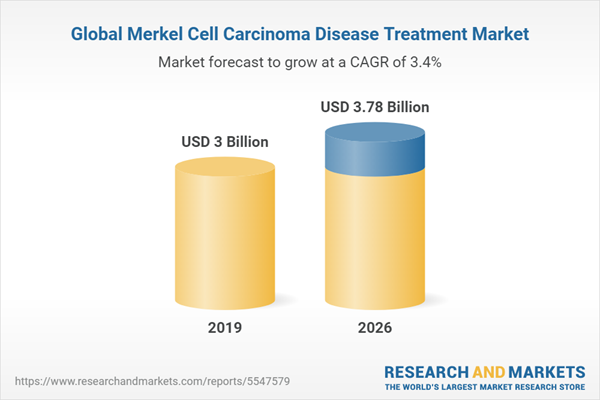
One of the key factors driving the Merkel cell carcinoma disease treatment market during the forecast period is the rising number of cases of Merkel cell carcinoma around the world. The increasing cases have influenced the demand for novel medications for Merkel cell carcinoma treatment. Furthermore, strong immunotherapy uptake and favorable reimbursement are regarded as encouraging indicators for Merkel cell carcinoma treatment growth. The market is expected to grow due to increased research and development initiatives for the treatment of Merkel cell carcinoma.
The growth of the Merkel cell carcinoma treatment market is being limited by high treatment costs, the cancellation of late-stage clinical trials, a lack of public awareness, and side effects during the projection period. Nausea, skin changes, diarrhea, painful sores in the throat and mouth, exhaustion, and dry mouth or thick saliva are all side effects of external radiation therapy. The radiation's negative effects are transient, although some uncommon significant side effects may become permanent. Radiation to the chest can sometimes cause lung damage, which can cause breathing problems and shortness of breath.
Key Developments
Nantkwest, Inc. (Immunitybio) announced the start of phase 2 clinical investigation on triple combination immunological therapy for Merkel cell cancer treatment in January 2019. KEYTRUDA was approved by the FDA in December 2018 for treating pediatric and adult patients with metastatic or recurrent locally advanced Merkel cell carcinoma. On July 9, 2019, The Patient Advocate Foundation, a non-profit organization, offered funds for MCC patients to assist with treatment travel expenditures. Once accepted, the financial fund will pay $450 in one-time help to cover the costs of transportation to and from treatment appointments. According to the Fred Hutchinson Cancer Research Centre, a multidisciplinary team from the University of Washington and the Fred Hutchinson Cancer Research Center was awarded a five-year, $12 million grant to study Merkel cell cancer on April 16th, 2019.
By therapy, the Merkel cell carcinoma disease treatment market is segmented into chemotherapy, radiation therapy, combination therapies, surgical excision, immunotherapy, and others. Etoposide, cisplatin, carboplatin, and topotecan are the different types of chemotherapy. Because of growing research and reimbursement, the chemotherapy segment is expected to dominate the market in the projected period. Pembrolizumab and avelumab are two types of immunotherapy drugs. Due to the approval of immune checkpoint inhibitors, immunotherapy is likely to increase at a high rate over the forecast period.
By test type, the Merkel cell carcinoma disease treatment market is segmented into computerized axial tomography scan, positron emission tomography scan, biopsy test, immunohistochemistry test, and others. PET scans are effective and can be used to assess the presence of illness or other disorders in organs and/or tissues.
By end-users, the Merkel cell carcinoma disease treatment market is segmented into hospitals, home care, surgical centers, specialty clinics, and others. Preference for hospitals is expected to fuel segment growth due to the availability of innovative technology and a large number of facilities available on a single site.
By geography, the Merkel cell carcinoma disease treatment market is segmented into five regions North America, Europe, South America, Middle East and Africa, and Asia Pacific regions. Because of the rising cases of skin cancer and favorable government regulations as well as modern healthcare facilities, North America will hold the largest market share for Merkel cell carcinoma therapy in the projected period, followed by Europe. Because of increased government programs and a rapidly aging population, Asia-Pacific has emerged as the fastest-growing regional category in terms of revenue. Furthermore, China and Australia are seeing rising Merkel cell carcinoma incidences, which is boosting the need for treatment in these countries.
COVID-19 Insights
Due to COVID-19 pandemic lockdown measures were placed, preventing patient movement and likely identification and overburdening healthcare systems. Smaller practices have closed completely, while larger firms are currently catering to emergencies. This, in turn, will have a major impact on the Merkel cell carcinoma market. The COVID 19 epidemic would lead to an extremely slow turnaround time for patients undergoing selected procedures.
Key Market Segments
By Therapy
- Chemotherapy
- Immunotherapy
- Surgical Excision
- Radiation Therapy
- Combination Therapy
- Others
By Test Type
- Computerized axial tomography scan
- Positron emission tomography scan
- Biopsy test
- Immunohistochemistry test
- Others
By End-Users
- Hospitals
- Homecare
- Specialty Clinics and Surgical Centers
- Others
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- United Kingdom
- Germany
- France
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- South Africa
- Others
- Asia Pacific
- China
- Japan
- India
- South Korea
- Others
Table of Contents
Companies Mentioned
- Merck KGaA
- Pfizer Inc.
- Takeda Oncology (Millennium Pharmaceuticals Inc.)
- Ono Pharmaceutical Co. Ltd
- Bristol-Myers Squibb Company
- OncoSec Medical Incorporated
- McKesson Corporation
- Amgen Inc,
- Immunity Bio (NantKwest)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 119 |
| Published | January 2022 |
| Forecast Period | 2019 - 2026 |
| Estimated Market Value ( USD | $ 3 Billion |
| Forecasted Market Value ( USD | $ 3.78 Billion |
| Compound Annual Growth Rate | 3.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |