Speak directly to the analyst to clarify any post sales queries you may have.
The market is witnessing steady growth due to the increased availability of funding from various public and private institutes. In addition, there is increased support from regulatory bodies for product approvals and providing fast-track designations to the products, which encourages vendors to manufacture the products at a faster rate.
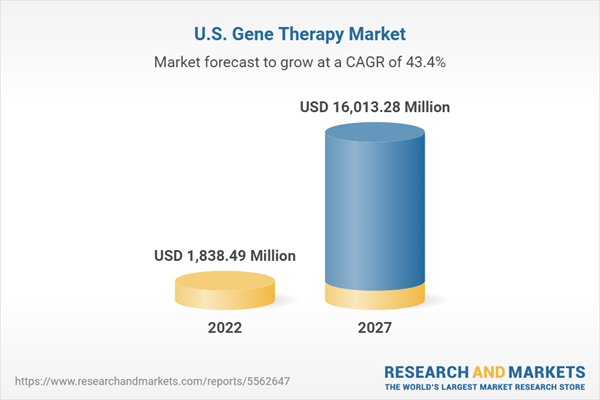
U.S. gene therapy market is expected to grow at a CAGR of 43.44% during 2022-2027
MARKET OPPORTUNITIES AND TRENDS
- Increasing Market Access for Gene Therapies
- Introduction Of Universal Car-T Therapy
- Increase In Strategic Acquisitions
- Increased Funding For R&D Activities of Gene Therapies
The US gene therapy market would realize an absolute growth of over 771.00% in terms of revenue between 2021 and 2027.
U.S. GENE THERAPY MARKET SEGMENTATION
Among product type segments, the CAR-T therapy segment dominates the US gene therapy market, accounting for a share of 51.96%, followed by non-CAR-T therapy with 48.04% in 2021.
Based on the vector type, the US gene therapy market is segmented into retroviral and others. The other segment includes adeno virus, adeno-associated virus, herpes simplex virus, and non-viral vectors. The retroviral segment accounted for a major share of 51.96% and others with 48.04% in 2021
Based on end-users, the market is segmented into hospitals, cancer care centers, and academic & research centers. The hospital segment dominated the market with a share of 56.45%, followed by cancer care centers with 35.87%.
Market Segmentation by Vector Type
- Retroviral
- Others
Market Segmentation by Product Type
- Car T Therapy
- Non-Car T Therapy
Market Segmentation by Indication
- Oncology
- Others
Market Segmentation by Gene Transfer
- Ex-Vivo
- In-Vivo
Market Segmentation by End User
- Hospitals
- Cancer Care Centers
- Academic & Research Centers
VENDOR ANALYSIS
The US gene therapy market is witnessing the high competition among the vendors. The market is characterized by the presence of various pharma and various small-scale companies. With the changing market landscape, the competition is turning intense due to some recent high-value M&As.
Key Vendors
- Amgen
- bluebird bio
- Bristol Myers Squibb
- Gilead Sciences
- Novartis
- F. Hoffmann-La Roche
Other Prominent Vendors
- 4DMT
- Abeona Therapeutics
- AnGes
- AskBio
- Astellas Gene Therapies
- Autolus Therapeutics
- Candel Therapeutics
- Castle Creek Biosciences
- Cellectis
- Evox Therapeutics
- Freeline Therapeutics
- Gene Biotherapeutics
- Generation Bio
- GenSight Biologics
- Helixmith
- Janssen Pharmaceuticals
- Kolon Tissue Gene
- Krystal Biotech
- MeiraGTx
- Orchard Therapeutics
- Pfizer
- Poseida Therapeutics
- Passage Bio
- RegenxBio
- Sana Biotechnology
- Sarepta Therapeutics
- StrideBio
- Solid Biosciences
- VBL Therapeutics
- Voyager Therapeutics
- Uniqure
THE REPORT INCLUDES:
1. The analysis of the U.S. Gene Therapy market size and growth rate for the forecast period 2022-2027.
2. It offers comprehensive insights into current industry trends, trend forecast, and growth drivers about the U.S. Gene Therapy market.
3. The report provides the latest analysis of market share, growth drivers, challenges, and investment opportunities.
4. It offers a complete overview of market segments and the regional outlook of the Gene Therapy market.
5. The report offers a detailed overview of the vendor landscape, competitive analysis, and critical market strategies to gain competitive advantage.
Table of Contents
Companies Mentioned
- Amgen
- bluebird bio
- Bristol Myers Squibb
- Gilead Sciences
- Novartis
- F. Hoffmann-La Roche
- 4DMT
- Abeona Therapeutics
- AnGes
- AskBio
- Astellas Gene Therapies
- Autolus Therapeutics
- Candel Therapeutics
- Castle Creek Biosciences
Methodology
Our research comprises a mix of primary and secondary research. The secondary research sources that are typically referred to include, but are not limited to, company websites, annual reports, financial reports, company pipeline charts, broker reports, investor presentations and SEC filings, journals and conferences, internal proprietary databases, news articles, press releases, and webcasts specific to the companies operating in any given market.
Primary research involves email interactions with the industry participants across major geographies. The participants who typically take part in such a process include, but are not limited to, CEOs, VPs, business development managers, market intelligence managers, and national sales managers. We primarily rely on internal research work and internal databases that we have populated over the years. We cross-verify our secondary research findings with the primary respondents participating in the study.

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 208 |
| Published | March 2022 |
| Forecast Period | 2022 - 2027 |
| Estimated Market Value ( USD | $ 1838.49 Million |
| Forecasted Market Value ( USD | $ 16013.28 Million |
| Compound Annual Growth Rate | 43.4% |
| Regions Covered | United States |
| No. of Companies Mentioned | 14 |