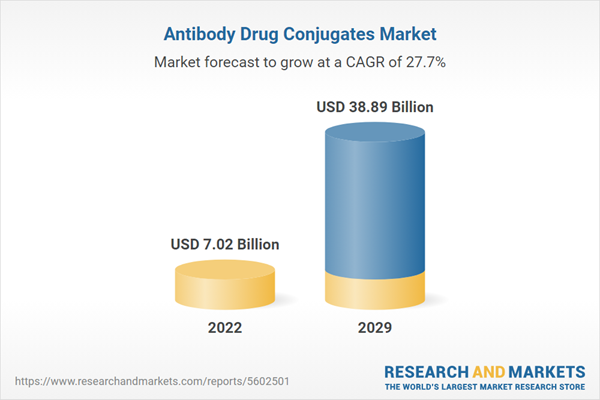
The global antibody drug conjugates market is projected to witness a CAGR of 27.7% during the forecast period to reach a total market size of US$38.885 billion by 2029, increasing from US$7.017 billion in 2022.
Antibody Drug Conjugates, or ADCs for short, are biological drugs designed as a targeted therapy for treating cancer. Antibody-drug conjugates provide sensitive differentiation between healthy and diseased tissue by combining the specific targeting of monoclonal antibodies with the cancer-killing capacity of cytotoxic medicines. ADC is a three-component system with a potent cytotoxin anticancer agent linked by a biodegradable linker to an antibody. The antibody attaches to certain markers on the cancer cell's surface (antigens or receptors). Within the cancer cell, the entire antibody-drug conjugate is internalized, where the linker is degraded and the active drug is liberated.The key growth factor driving the antibody-drug conjugate market is the growing prevalence of cancer. Cancer affects millions of lives every year and is increasing. The need to develop an effective treatment is higher than ever before. Further, increasing health consciousness amongst individuals helps identify cancer in its early stages, which can be treated on time without any further spread of cancer to other parts of the body. Moreover, growing healthcare expenditure on cancer treatment and rising R&D in ADCs play an important role in the growth of the global antibody-drug conjugate market. Furthermore, the global trend toward targeted cancer therapeutics, as well as increased research and development on ADCs, are significantly contributing to the market. Targeted therapy helps to eliminate cancer cells without actually harming healthy cells.
MARKET DRIVERS
Patients with Cancer Are Increasing
Patients with cancer have seen an upward trend. Globally, cancer is the second largest cause of death. According to the World Health Organization, 9.6 million people are estimated to die due to cancer worldwide in 2018. Around 400,000 children develop cancer each year. With increasing awareness of cancer and rising, medical facility detection of cancer in its early stages, early detections help eliminate it before some serious damage is done. An increasingly sedentary lifestyle and increasing consumption of junk food, tobacco, and alcohol are some of the prevalent factors causing cancer. Around one-third of deaths are from cancer due to tobacco and alcohol use, obesity, low fruit and vegetable intake, and lack of physical activity. In 2020, the most common cause of death was lung cancer, followed by colon and rectum cancer, and liver cancer. Accounting for 180 million deaths by lung cancer, 9.35 thousand deaths due to colon and rectal cancer, and 830 thousand deaths due to liver cancer. Breast cancer is another of the most common types of cancer. With early detection and proper treatment, deaths could be prevented. Between 30-50% of cancer cases can currently be prevented.ADC research and development is becoming increasingly extensive around the world.
With the increasing development in research and development by various companies to find an effective cancer treatment, more and more companies are investing in R&D to develop such drugs. With a deeper understanding and the advent of new technology, the scope of R&D in ADCs has further increased. Moreover, the approval rate for U.S. Food and Drug Association (FDA) products has increased significantly. In the year 2021, 12 ADC drugs have been approved. Further, deep research on interactions between ADCs and the immune system for potential synergistic therapeutic effects that can be used to improve the drug is taking place.MARKET RESTRAINT:
Expensive Treatment
Cancer treatments come with a hefty price tag., due to extensive research, licensing, patents, and manufacturing costs of drugs. According to the World Health Organization (WHO), the total annual economic cost of cancer in 2010 was estimated at US$ 1.16 trillion. Antibody-drug conjugates can be an expensive treatment for cancer patients, with a high level of time and money invested in R&D adding to the cost. ADCs also have a high manufacturing cost, which adds up to the final cast. The dominant cost in the manufacturing process of raw materials. Depending on the nature of the toxins, the drug-linker raw material can cost between $200-$2000 per gram. Furthermore, a small batch of doses produced per year is not able to reach economies of scale. Further, strict regulation from medical and drug authorities adds to the cost.Key Developments:
- July 2023- ImmunoBiochem has signed a multi-target license and option agreement with ImmunoGen to advance antibody-drug conjugates (ADCs). The collaboration will combine ImmunoGen's linker and payload technologies with ImmunoBiochem's antibodies against specific targets. ImmunoBiochem will receive an upfront fee in exchange for an exclusive license to existing antibodies. The collaboration will focus on preclinical activities, with ImmunoGen responsible for clinical development and commercialization.
- July 2023- BeiGene and DualityBio have agreed to acquire an exclusive option for a global clinical and commercial license for an investigational, preclinical ADC therapy for patients with solid tumors. DualityBio will receive an upfront payment and additional payments based on milestones, totaling up to $1.3 billion. BeiGene will hold global rights, while DualityBio will conduct research and support future IND filings.
Market Segmentation:
By Product Type
- Adcetris
- Kadcyla
- Other Product Types
By Target Type
- Cd30 Antibodies
- Her2 Antibodies
- Others
By Technology
- Cleavable Linker
- Non-Cleavable Linker
By Application
- Breast Cancer
- Blood Cancer
- Urinary Tract/Bladder Cancer
- Cervical And Head & Neck Cancer
By End-User
- Hospitals
- Clinics
- Others
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- United Kingdom
- Germany
- France
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- Japan
- India
- South Korea
- Others
Table of Contents
1. INTRODUCTION
2. RESEARCH METHODOLOGY
3. EXECUTIVE SUMMARY
4. MARKET DYNAMICS
5. GLOBAL ANTIBODY DRUG CONJUGATES MARKET, BY PRODUCT TYPE
6. GLOBAL ANTIBODY DRUG CONJUGATES MARKET, BY TARGET TYPE
7. GLOBAL ANTIBODY DRUG CONJUGATES MARKET, BY TECHNOLOGY
8. GLOBAL ANTIBODY DRUG CONJUGATES MARKET, BY APPLICATION
9. GLOBAL ANTIBODY DRUG CONJUGATES MARKET, BY END-USER
10. GLOBAL ANTIBODY DRUG CONJUGATES MARKET, BY GEOGRAPHY
11. COMPETITIVE ENVIRONMENT AND ANALYSIS
12. COMPANY PROFILES
Companies Mentioned
- The Linde Group
- Astrazeneca
- Pfizer, Inc
- Seagen, Inc.
- GSK Plc
- Takeda Pharmaceutical Company Ltd.
- F. Hoffmann-La Roche Ltd.
- Novartis
- Gilead Sciences, Inc.
- Sanofi S.A.
- Astellas Pharma
- Daiichi Sankyo Company Ltd.
- Genentech Inc.
- Immunogen Inc.
- Lantheus
- Bayer Healthcare Pharmaceuticals
- Astellas Pharma
- Synthon Holding BV
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 210 |
| Published | February 2024 |
| Forecast Period | 2022 - 2029 |
| Estimated Market Value ( USD | $ 7.02 Billion |
| Forecasted Market Value ( USD | $ 38.89 Billion |
| Compound Annual Growth Rate | 27.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |