The COVID-19 pandemic has impacted market growth. The high burden of coronavirus has increased the demand for multiplex assays as multiplex tests were used for the diagnosis of COVID-19. For instance, according to an article published in Aging Journal, in December 2021, the multiplex RT-PCR developed on the LabTurbo AIO 48 open system was used to identify SARS-CoV-2 and other widespread respiratory viruses. It has been observed that the multiplex RT-PCR used on the LabTurbo AIO 48 open platform provided highly sensitive, robust, and accurate results and enabled high-throughput detection of B.1.1.7, influenza A/B, and RSV with short turnaround times. Moreover, the utilization of multiplex assays based on different technology has increased for detecting various chronic and infectious diseases, which has further propelled the market growth.
Factors such as the increasing adoption of personalized-precision medicine, the rising burden of chronic and infectious diseases, and the distinct advantages of multiplex assays over other singleplex assays are expected to boost market growth over the forecast period.
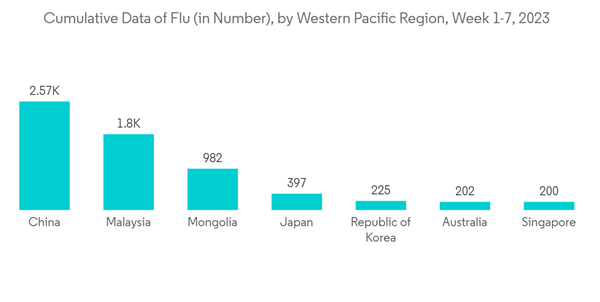
The growing prevalence and incidence of chronic and infectious diseases among the population raise the need to diagnose as well as monitor the progression of the disease of certain viruses. This is anticipated to fuel the demand for multiplex assays over the forecast period. For instance, the data published by WHO on the 'Bi-Weekly Influenza Situation', in March 2023, stated that in China, during week 7 of 2023, sentinel hospitals in the northern provinces reported an influenza-like illness (ILI) of 1.9%, up from 1.4% the week before. Also, from the same source, in April 2022, about 169 cases of acute hepatitis of unknown origin had been recorded from around 11 countries in the WHO European Region and one nation in the WHO Region of the Americas, raising concerns about the disease. Similarly, according to 2022 statistics published by the International Diabetes Federation, about 537 million people were living with diabetes in 2021, globally, and this number is projected to reach 643 million and 784 million by 2030 and 2045, respectively. Thus, such an increase in the diabetic population is likely to raises the demand for multiplex assays to facilitate the screening of diabetes which is expected to bolster the market growth.
Additionally, the increasing use of multiplex assays is another factor driving the market growth. For instance, as per an article published in Biology Journal, in November 2021, the binding patterns of two CCR2-specific and five CCR5-specific antibodies, in a multiplex immunoassay, to the 12-domain-swapped mutants were examined. It has been observed that the multiplex immunoassay helps speed up the discovery of therapeutic or diagnostic antibodies by enabling high-throughput screening of antibodies with the required specificity and possible function at single-residue resolution in a single tube. Thus, such benefits offered by multiplex assays increase their adoption over the forecast period, thereby propelling the market growth.
Furthermore, several advantages offered by multiplex assays such as increased efficiency at a reduced expense, greater output per sample volume ratios, and higher throughput predicting more resolute, detailed diagnostics and facilitating personalized medicine, over singleplex assays make its more preferred choice for detecting multiple biomarkers in chronic and infectious diseases. This is also anticipated to augment the market growth over the forecast period.
Moreover, the rising product launches by the companies increase the availability of novel multiplex assay kits and systems in the market, hence contributing to market growth. For instance, in May 2022, QuantuMDx Group Limited launched a new multiplex respiratory panel test, Q-POC SARS-CoV-2, Flu A/B & RSV Assay. The multiplex capabilities provide accurate PCR results within 30 minutes. Also, in June 2021, Eurofins Technologies launched a new multiplex GSD NovaType III SARS-CoV-2 RT-PCR assay for the rapid detection of SARS-CoV-2 Variants of Concern including B.1.617, B.1.427/B.1.429, B.1.351, or P.1. The assay facilitates the simultaneous differentiation from the S gene E484 wildtype variant and the identification of the pertinent mutations E484Q, E484K, and L452R in a reaction.
Therefore, owing to the aforementioned factors, the studied market is expected to grow over the forecast period. However, the high cost of equipment coupled with the cumbersome complexity of procedures as well as the regulatory constraints for product approvals are likely to hinder the growth of the multiplex assay market over the forecast period.
Multiplex Assays Market Trends
Multiplex Real-Time PCR Segment is Expected to Hold a Major Share in the Multiplex Assays Market
Multiplex polymerase chain reaction (PCR) is the process in which more than one target sequence can be amplified by including more than one pair of primers in the reaction. This process amplifies DNA in samples using multiple primers and a temperature-mediated DNA polymerase in a thermal cycler. Some of the advantages of multiplex qPCR include higher throughput, lower reaction costs, and preservation of scarce sample materials. Besides, a set of primers can be used as an internal control in multiplex PCR which has the benefit of eliminating the risk of false positives or negatives.The multiplex polymerase chain reaction segment is expected to witness significant growth over the forecast period owing to the factors such as rising R&D activities, and various strategies such as product launches adopted by key market players.
The use of multiplex assays in determining pathogenic mutations in reproductive genetic testing is increasing. For instance, according to an article published in Nature Journal, in January 2022, a carrier screening assay, a type of reproductive genetic testing for couples for assessing the risk of passing on certain genetic conditions to offspring, for 448 pathogenic mutations was developed using capillary electrophoresis-based multiplex PCR technology. The capillary electrophoresis-based multiplex PCR assay demonstrated sensitivity, specificity, and accuracy of 97.4%, 100%, and 99.6%, respectively, in detecting the specific variants. Thus, the high accuracy offered by multiplex assays in genetic testing is anticipated to fuel the segment growth.
Additionally, as per an article published in PLOS One, in April 2021, a multiplex real-time reverse transcription polymerase chain reaction (rRT-PCR) assays was developed for the detection of SARS-COV-2, that simultaneously targets two viral genes (RdRP and E) and a human gene (RP) as an internal control by using the Applied Biosystems 7500 Fast Real-Time PCR instrument. It has been observed that the multiplex rRT-PCR method used for the detection of SARS-CoV-2 has provided an accurate, reliable, and easy-to-use SARS-CoV-2 diagnostic test. As per the same source, the assay is efficient to detect three genes in the same reaction tube and allows working with large numbers of patients using less PCR reagent. Thus, such advantages offered by the multiplex PCR method are expected to increase its adoption in detecting other viral infections, hence contributing to segment growth.
Furthermore, the rising new product launches increase the availability of novel products in the market which is also contributing to segment growth. For instance, in July 2022, Bio-Rad Laboratories launched CFX Opus Deepwell Real-Time PCR Detection System to support researchers in developing nucleic acid detection assays. The system can multiplex up to five targets simultaneously and supports fluorescence resonance energy transfer applications. Also, in May 2022, Cipla Limited in partnership with Genes2Me Pvt. Ltd launched an RT-Direct multiplex COVID-19 RT PCR Test kit. This launch assists the company to expands its diagnostics offering to bring more advanced and innovative products.
Therefore, owing to the aforementioned factors, the studied segment is expected to grow over the forecast period.
North America is Expected to Have the Significant Market Share Over the Forecast Period
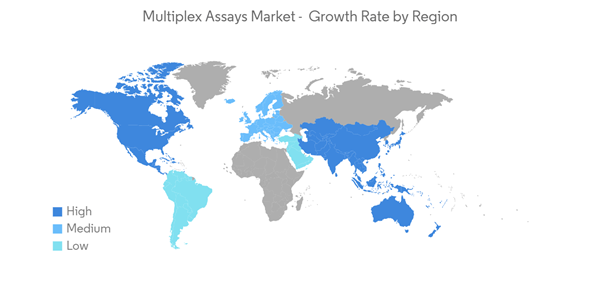
North America is expected to hold a significant share in the market over the forecast period owing to the factors such as the growing burden of chronic diseases such as HIV, CNS disorders, gastrointestinal disorders, and others along with the rising adoption of multiplex assays over single assays, increasing product approvals, and investments.The rising prevalence of various diseases such as cancer, cardiovascular diseases, influenza, Alzheimer's disease, and others among the population raises the demand for diagnosing multiple biomarkers for the detection of diseases. This in turn is anticipated to boost the market growth. For instance, according to the 2023 statistics published by ACS, about 19,58,310 new cancer cases are expected to be diagnosed in the United States in 2023. Additionally, as per 2022 statistics published by the Canadian Cancer Society, about 233,900 people were diagnosed with cancer in Canada in 2022. Also, according to the 2022 statistics published by AHA, more than 130 million adults in the United States are expected to have some type of heart disease by 2035. Thus, the expected increase in the population suffering from cancer and heart diseases is anticipated to fuel the demand for multiplex assays to simultaneously analyze multiple biomarkers from a single sample, thereby contributing to the market growth.
Furthermore, with the increasing company focus on adopting various key strategies such as collaboration, partnerships, agreements, and product launches, the studied market is expected to grow over the forecast period. For instance, in April 2022, Bruker Corporation launched a unique LiquidArray multiplex PCR assay that detects seven major pathogens causing Sexually Transmitted Infections (STIs). Also, in January 2022, Health Canada approved Seegene's Allplex SARS CoV-2 FluA/FluB/RSV assay, a multiplex real-time-PCR assay designed to facilitate simultaneous amplification and differentiation of respiratory symptoms.
Therefore, owing to the aforementioned factors, the studied segment is expected to grow over the forecast period.
Multiplex Assays Industry Overview
The multiplex assay market is moderately competitive in nature. The companies are adopting various key strategies such as collaborations, partnerships, agreements, acquisition and new product launches to withhold their market position. Some of the key players in the market are Thermo Fisher Scientific, Inc., Abcam plc, Merck KGaA, Perkinelmer, Inc., Bio-Rad Laboratories, Inc., Quansys Biosciences Inc., and Promega Corporation among others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abcam plc.
- Bio-Rad Laboratories, Inc.
- Biomerieux SA
- Hologic Corporation
- Luminex Corporation
- Merck KGaA
- Perkin Elmer Inc.
- Promega Corporation
- Quansys Biosciences Inc.
- Seegene Inc.
- Thermo Fisher Scientific
- Qiagen