Key Highlights
- Although COVID-19 led to a shortage in essential diagnostic tests during the early pandemic, multiplex diagnostics have played an important role in providing quick results for COVID-19 diagnostics. However, the launch of products is propelling the growth of the market. For instance, in February 2022, Avellino Labs USA Inc. launched AvellinoCoV2 - Respiratory Test, a multi-panel RT-PCR-based virus assay that accurately detects four viral infections in one patient sample - COVID-19, respiratory syncytial virus (RSV), influenza A and influenza B. Thus, COVID-19 has slightly declined during the early pandemic due to lockdown restrictions. However, the market was positively impacted during the late pandemic due to a rise in testing for RSV and saw a significant surge post-pandemic due to the launch of new tests, and it is expected to boost the market's growth over the period.
- The factors propelling the studied market growth are the growing burden of respiratory syncytial virus infections globally, rise in investments, technological advancements in the development of RSV diagnostic tests, product approvals and launches, and other key strategies adopted by the market players.
- According to the Center for Disease Control and Prevention (CDC) update in July 2023, the overall rate of RSV-associated hospitalizations was 50.9 per 100,000 people in the United States in the 2022-2023 season. Such a huge rate of hospitalization is anticipated to increase the adoption of testing for RSV infections, driving the market growth.
- The launch of products for the effective diagnosis of RSV infections is expected to boost the studied market growth due to the increased availability of products in the market. For instance, in November 2021, QIAGEN launched the QIAstat-Dx Respiratory 4 Plex Flu A-B/RSV/SARS-CoV-2 test for the QIAstat-Dx system to quickly identify whether patients have common seasonal respiratory infections or SARS-CoV-2. Additionally, in September 2021, Roche launched three molecular polymerase chain reaction (PCR) diagnostic test panels to detect and differentiate common respiratory pathogens at the same time, which include influenza A, influenza B, and respiratory syncytial virus (RSV); adenovirus (ADV), human metapneumovirus (hMPV) and enterovirus/rhinovirus (EV/RV); and parainfluenza 1, 2, 3 and 4.
- Moreover, key initiatives adopted by key market players are expected to propel market growth significantly. In February 2022, Seegene Inc. signed a supply deal with the Ministry of Health of Brazil to deliver four million Allplex SARS-CoV-2/FluA/FluB/RSV Assay, capable of identifying respiratory viruses, including COVID-19, Flu A/B as well as RSV in a single test. Such steps will boost the studied market growth during the study period due to the rise in adoption.
- Therefore, owing to factors such as the rising incidence of RSV and key initiatives adopted by the market players, the studied market is expected to grow significantly during the study period. However, strict regulatory norms and low detection limits of immunoassays are expected to hinder the market growth during the study period.
Respiratory Syncytial Virus (RSV) Diagnostic Market Trends
Rapid Antigen Detection Tests Segment is Expected to Account for the Significant Market Share During the Forecast Period
- A rapid antigen test (RAT), sometimes called a rapid antigen detection test (RADT) or rapid antigen test (ART), is a rapid diagnostic test suitable for point-of-care testing that directly detects the presence or absence of an antigen.
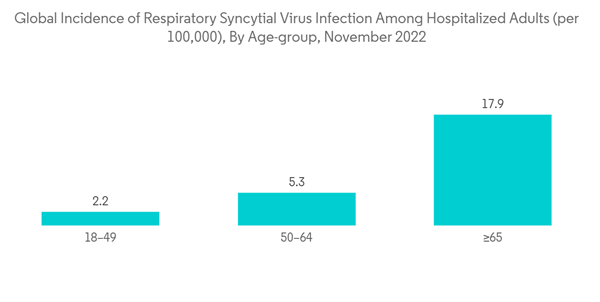
- These tests provide results within 15 to 30 minutes, require minimal training or infrastructure, and have significant cost advantages. RATs are based on the principle of antigen-antibody interaction. Antigen-antibody reactions are a mainstay for the rapid detection of proteins. Antibodies recognize proteins based on their structure as well as their content. They can be very specific, binding to only a small part of an antigen and discriminating between highly similar antigens. Older adults are at greater risk than young adults for serious complications from RSV because of their poor immune systems.
- The rising research and development for the development of rapid antigen tests, rising product launches, and key initiatives taken by market players and the government are expected to boost the segment growth during the study period. According to the study published in the Journal of Infectious Diseases in February 2022, the positive percentage agreement of Xpert Xpress Flu/RSV, a rapid antigen test, compared to routine RT-PCR, is high for RSV detection in home-dwelling older adults. The assay is fast and easy to use at the point of care. Such advantages of RATs will propel the segment's growth.
- Furthermore, the rising investments to boost product development and business expansions in the studied segment will drive the segment growth. For instance, in March 2021, the National Institute of Health launched a Rapid Acceleration of Diagnostics Tech (RADx) initiative, with a total investment of USD 29.3 million focusing on increasing testing capacity at the point-of-care settings. Further, through this investment, Meridian Biosciences is developing a respiratory panel that includes SARS-CoV-2, Influenza A/B, and RSV in one test. Additionally, in March 2021, Quidel Corporation opened a new manufacturing facility in Carlsbad, California, that will be dedicated to the production of the QuickVue line of products, which includes a rapid test for Influenza, RSV, Strep A, and other diseases and conditions.
- Thus, due to the above-mentioned factors, the studied segment is expected to contribute to the significant growth of the market.
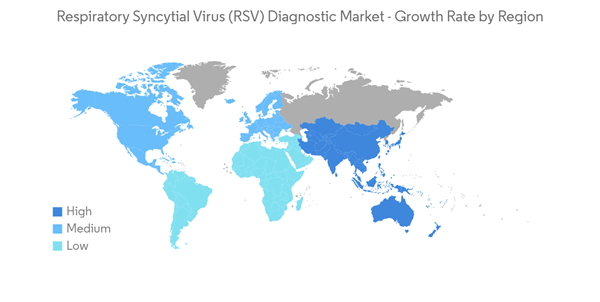
North America is Expected to Hold a Significant Share in the Market and Expected to do Same Over the Forecast Period
- The primary driving factors for the growth of the North American respiratory syncytial virus (RSV) market are the growing burden of cold and pneumonia in the region, growing research and development activities, supporting government initiatives such as approvals for RSV diagnostics and initiatives taken by the key market players.
- The United States within North America is expected to hold a significant share of the studied market during the study period due to the rising hospitalizations for RSV, rising approval of products from regulatory agencies, and the strong foothold of key market players. For instance, according to the Centers for Disease Control and Prevention, in June 2021, in the United States, RSV accounts for approximately 58,000 hospitalizations among children younger than 5 years old and 177,000 hospitalizations among adults aged 65 years or older every year. Thus, the rising hospitalizations for RSV infections are anticipated to boost the demand for RSV diagnostics, driving market growth.
- Moreover, product approvals in the regions will also boost the diagnostic procedures in the region. For instance, in May 2022, the United States Food and Drug Administration (US FDA) authorized the LabCorp Seasonal Respiratory Virus RT-PCR DTC test for use without a prescription by individuals with symptoms of respiratory viral infection consistent with COVID-19. Additionally, in December 2021, Applied BioCode received Emergency Use Authorization (EUA) from the US FDA for its BioCode CoV-2 Flu Plus Assay. This PCR-based, multiplex molecular diagnostic assay can simultaneously detect and differentiate between SARS-CoV-2, Influenza A with subtypes (seasonal H1, 2009 H1N1, H3, Influenza B), and Respiratory Syncytial Virus (RSV) in nasopharyngeal swab specimens.
- Furthermore, in January 2022, Under Health Canada's Interim Order, Seegene Inc. received approval for its Allplex SARS CoV-2 FluA/FluB/RSV Assay. The Allplex SARS CoV-2 FluA/FluB/RSV Assay from Seegene is a multiplex real-time PCR assay that allows for both amplification and differentiation of respiratory symptoms. It can distinguish between Influenza A, B, RSV, and COVID-19 in a single test.
- Therefore, the key strategies adopted by the market players, along with the rising hospitalization due to RSV in the region, are expected to lead to lucrative growth of the market in North America.
Respiratory Syncytial Virus (RSV) Diagnostic Industry Overview
The respiratory syncytial virus (RSV) diagnostic market is moderately competitive, with the presence of several players. The factors owing to the competition include strategies such as acquisitions, partnerships, distribution agreements, and others that are readily adopted by key market players to boost the market. Some players operating in the market are BioMerieux SA, Becton, Dickinson and Company, Abbott, F. Hoffmann-La Roche Ltd, ThermoFisher Scientific Inc., and others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- bioMerieux SA
- Becton, Dickinson and Company
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- ThermoFisher Scientific Inc.
- Luminex Corporation
- Qiagen
- Hologic, Inc.
- DiaSorin Inc.
- Bio-Rad Laboratories, Inc.
- Merck KGaA
- Sartorius AG