The COVID-19 pandemic significantly impacted the androgen deprivation therapy market, as cancer patients, especially prostate cancer patients, were more susceptible to coronavirus than individuals without cancer. The high susceptibility was due to the immunosuppressive state caused by malignancy and anticancer treatment. Moreover, according to the data from Cancer Treatment and Research Communications published in January 2022, it was found that during the first COVID-19 wave, there was a 17% decrease in prostate cancer diagnoses in the Netherlands. The reduction was most noticeable among older people and those with low-risk localized cancer. With the decrease in the diagnosis of cancers, the use of androgen deprivation therapy (ADT) was hampered. However, the market reached its pre-pandemic nature regarding the utilization of ADT among cancer patients, which is believed to boost the market growth in the coming years.
The key factors propelling the market growth are the rising burden of prostate cancer, increasing research and development activities, advancements in treatment technology for effective treatment of prostate cancer, rising product approvals, and strategic initiatives adopted by key market players.
Strategies such as partnerships, distribution agreements, mergers, and acquisitions adopted by key market players are expected to boost market growth. For instance, in May 2022, Myovant Sciences and Accord Healthcare, Ltd. entered into an exclusive license agreement for Accord to commercialize Relugolix for the treatment of advanced hormone-sensitive prostate cancer under the trade name ORGOVYX (Relugolix, 120 mg) in the European Economic Area, United Kingdom, Switzerland, and Turkey. Such partnerships will expand the accessibility of ORGOVYX across geographies, thereby leading to lucrative market growth.
Furthermore, achieving milestones in the drug development cycle will also fuel market growth. For instance, in May 2022, Bayer reported that the United States Food and Drug Administration (FDA) had accepted a supplemental New Drug Application (sNDA) and granted Priority Review for the oral androgen receptor inhibitor (ARi) darolutamide in combination with docetaxel for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC).
Therefore, owing to the factors above, the studied market is expected to grow significantly during the study period. However, limited reimbursement policies and increasing side effects are expected to hinder the market growth during the study period.
Androgen Deprivation Therapy Market Trends
Antiandrogen Sub Segment Within Drug Class Segment is Expected to Witness a Strong Growth Over the Forecast Period
Anti-androgens are drugs that connect to the androgen receptors, inhibiting the androgens from causing tumor growth. Anti-androgens are also sometimes called androgen receptor antagonists. Antiandrogens drug include Flutamide (Eulexin), Bicalutamide (Casodex), Nilutamide (Nilandron), Enzalutamide (Xtandi), apalutamide (Erleada) and darolutamide (Nubeqa).Factors such as rising incidences of prostate cancer globally, product approvals, growing geriatric population, increasing research and development activities, and strategies adopted by key market players such as partnerships will effectively contribute to the market growth.
The growing research activities are contributing significantly to the segment's growth. For instance, in December 2021, Bayer published the results from the Phase III ARASENS trial, which investigated the use of the oral androgen receptor inhibitor (ARi) Nubeqa (darolutamide), an anti-androgen, in metastatic hormone-sensitive prostate cancer (mHSPC). The company stated that darolutamide, in combination with docetaxel and androgen deprivation therapy (ADT), significantly increased overall survival compared to docetaxel and ADT.
Additionally, in September 2021, Astellas Pharma Inc. and Pfizer Inc. stated that XTANDI (enzalutamide), an anti-androgen, improved overall survival in men with metastatic hormone-sensitive prostate cancer, which is proved through the ARCHES study. Such studies highlight anti-androgen efficacies in treating prostate cancer in terms of increased overall survival rate, which boosts anti-androgen application, driving the market.
Furthermore, in February 2021, Bayer, in partnership with Orion Corporation, expanded the global clinical development program for the oral androgen receptor inhibitor (ARi) Nubeqa (darolutamide) in the area of prostate cancer. The company initiated a Phase III study, ARANOTE. It will evaluate the compound in addition to androgen deprivation therapy (ADT) versus placebo plus ADT in men with metastatic hormone-sensitive prostate cancer (mHSPC). Such strategies will allow companies to leverage others' strengths and develop an advanced product in prostate cancer, thereby leading to lucrative market growth.
Thus, due to the factors mentioned above, the studied segment is expected to contribute to the significant growth of the market.
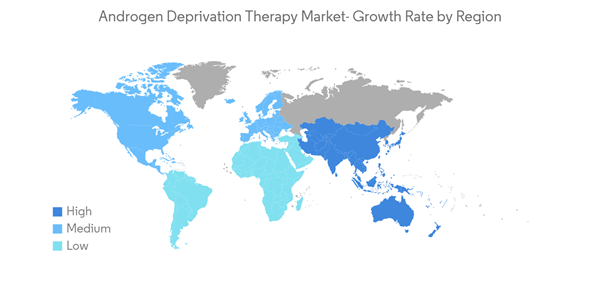
North America is Expected to Hold a Significant Share in the Market and Expected to do Same in the Forecast Period
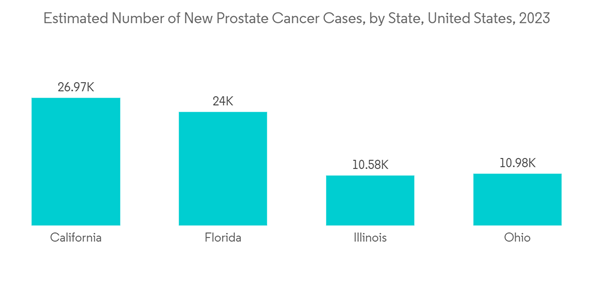
The primary driving factors for the growth of the North American androgen deprivation therapy (ADT) market are the rising prevalence of prostate cancer, the growing geriatric population, product launches, and several initiatives and investments made by key market players.The United States within North America is expected to hold a significant share of the studied market during the study period. Prostate cancer is the most common cancer in American men. The American Cancer Society estimated that in the United States in 2023, about 288,300 new cases of prostate cancer are expected to be reported. Thus, the country's huge burden of prostate cancer is expected to increase the demand for its therapeutics. It can suppress the testosterone formed, contributing to market growth.
The above source also suggests prostate cancer is more likely to develop in older men. About 6 cases in 10 are diagnosed in men 65 or older, and it is rare in men under 40. The average age of men at diagnosis is about 66. Thereby, the growing elderly population in the region will also increase the incidence rate of prostate cancer, propelling the market.
The rising research and development studies proving the efficacies of androgen receptor inhibitors in treating pancreatic cancer will contribute to market growth. For instance, in February 2021, the Janssen Pharmaceutical Companies of Johnson & Johnson released results from the final analysis of the Phase 3 TITAN study. It stated that ERLEADA, in combination with ADT, provided a statistically significant improvement in overall survival rate with a 35% reduction in risk of death versus ADT alone. Such technological improvement in treatment procedures is expected to drive market growth.
Additionally, in January 2021, the US FDA approved Relugolix (Orgovyx) for treating adults with prostate cancer as it is more effective at reducing testosterone levels in men with advanced prostate cancer than another commonly used treatment, leuprolide (Lupron). Therefore, the studied market is expected to lead to lucrative growth in North America due to the abovementioned factors.
Androgen Deprivation Therapy Industry Overview
The androgen deprivation therapy market is moderately competitive. The strategies such as mergers and acquisitions and partnerships adopted by the key players will boost the market. The major players in the market are Johnson and Johnson, AstraZeneca, Bayer AG, Astellas Pharma, Tolmar Pharmaceuticals Inc., and others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Johnson and Johnson
- Astrazeneca
- Bayer AG
- Astellas Pharma Inc.
- AbbVie Inc.
- Tolmar Pharmaceuticals, Inc.
- Verity Pharmaceuticals Inc.
- Foresee Pharmaceuticals Co., Ltd.
- Myovant Sciences GmbH
- Ferring B.V.
- Viatris
- Bristol-Myers Squibb Company