Key Highlights
- The massive volume of COVID-19 patients and the lockdown disrupted the diagnosis and treatment of non-COVID diseases. The increasing adoption of devices that were used to treat COVID-19 patients in intensive care consequently decreased the adoption rate of other devices. This was mainly due to the postponement or cancellation of procedures and surgeries during the pandemic.
- For instance, according to the study published in the Clinical Kidney Journal in April 2022, an increase in the use of central venous catheters (CVCs) was observed in comparison to prosthetic arteriovenous fistula (AFVs). The study's main objective was to investigate how the COVID-19 pandemic affected the development of vascular access devices, central venous catheter (CVC)-related hospitalizations, and complications in hemodialysis patients dialyzed in 16 Spanish hemodialysis units across three distinct geographic areas. When compared to a pre-COVID-19 period, a decrease in hemodialysis sessions was observed. However, the number of COVID-19 patients decreased in the post-pandemic phase, which led to the resumption of vascular surgeries and treatment, which enabled the market to grow at a steady pace in Europe. Thus, the COVID-19 pandemic adversely impacted the market in its preliminary phase in Europe. However, the market resumed its normal growth pace in the post-pandemic phase with the resumption of vascular disease treatment and increased vascular procedures in Europe and is expected to continue the upward trend over the forecast period.
- Factors such as the growing prevalence of chronic disease, an increasing number of chemotherapy procedures with high hospitalization rates, rising use of vascular access devices among pediatric patients, and growing initiatives from the European authorities are likely to drive the market growth positively.
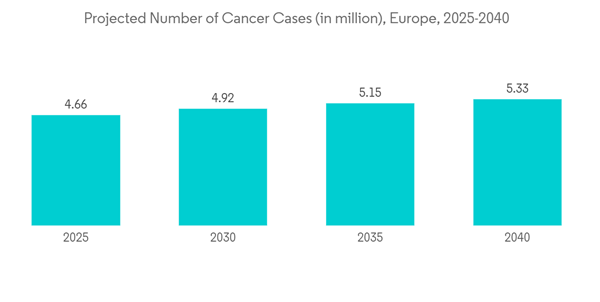
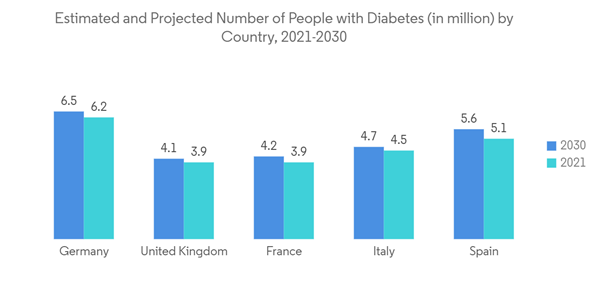
- Over the past decade, there has been a rise in the prevalence of several chronic diseases that need vascular access treatment. Some of the major chronic diseases include cancer, kidney failure, and heart disease, among others within the European region. For instance, according to the research study published by the NCBI in October 2021, a high burden of peripheral vascular diseases was observed in the European region in 2021. Such an increase in the patient population is ultimately boosting the growth of surgeries within the region, thereby driving the demand for vascular access devices. Furthermore, according to the International Diabetes Federation (IDF), in February 2022, over 4,470,300 adults were reported to have diabetes out of the total adult [population in Italy, which was equivalent to a prevalence rate of 9.9%. It also stated that over 1.2 million children and adolescents aged 19 years or less were reported to have type 1 diabetes. Also, as per the data published by the International Agency for Research on Cancer (IARC) in 2023, the cases of cancer are expected to increase in Germany in the coming years, with 21.9 million cancer cases in 2025 and 24.6 million cancer cases in 2030. Such an increasingly high burden of cancer is anticipated to bolster the volume of chemotherapy, which in turn is expected to increase the demand for vascular access devices for the administration of drugs, fluid, and nutrition. Thus, considerable market growth is expected over the forecast period.
- Moreover, the recent developments by the market players have increased the penetration of vascular access devices in the European region; for instance, in September 2022, Delta Med Group, an Italy-based vascular access device for oncology and analgesic therapies, signed a strategic partnership with Pentaferte to compete in the European infusion medical device market. Similarly, in September 2021, Medtronic PLC received CE Mark approval for its radial artery access portfolio, which includes the Rist 079 Radial Access Guide Catheter and Rist Radial Access Selective Catheter. Also, in July 2021, Vivasure Medical launched its development program for PerQseal Blue, the new investigational sutureless and fully absorbable large-bore venous vessel closure following percutaneous cardiovascular procedures. PerQseal is currently available to physicians in Europe for use in novel transcatheter endovascular procedures that require large-bore arterial vessel access.
- Therefore, owing to the increasing burden of chronic diseases such as cancer, cardiovascular diseases, and diabetes and strategic initiatives by the key players, the market is expected to show significant growth over the forecast period.
- However, risks associated with catheter usage, stringent regulations, and product recalls are expected to hinder the market's growth over the forecast period.
Europe Vascular Access Device Market Trends
The Administration of Drugs Segment is Expected to Garner Significant Share of the Market
- The segment includes the vascular access devices which are used for the administration of drugs. Vascular access devices are used for various functions, including administering drugs, drawing blood, and specialized treatments like hemodialysis. Vascular access catheters can be inserted either peripherally in a patient's arm or centrally, such as in the patient's jugular.
- The administration of drugs segment is expected to grow in the forecast period with the factors owing to the high usage of vascular access devices for drug administration for the treatment of infections, cancer, and other lifestyle diseases.
- Research studies have highlighted the safety and efficacy of vascular access devices for drug administration in patients, which are expected to boost the adoption rate. For instance, a study published in the International Journal of Clinical Practice in December 2021 stated the widespread use of vascular access devices has offered the safe administration of drugs, electrolytes, fluids, parenteral nourishment, and blood products in Europe. The study further mentioned that over 60% to 90% of patients admitted to hospitals were prescribed an intravenous (IV) vascular access device.
- Furthermore, the burden of certain chronic diseases is high, which is also anticipated to increase the demand for vascular access devices for drug administration, boosting the segment's growth, for instance, as per the data published by the European Kidney Alliance in 2021, over 75.0 million people suffer from chronic kidney disease (CKD) in Europe. As kidney transplants and dialysis are the only choices of treatments for most of the patients suffering from CKD, the demand for vascular access devices in the patient population is likely to boost over the studied period.
- Therefore, the high burden of chronic diseases and the high efficacy and safety offered by vascular access devices are likely to boost the growth of the administration of drugs segment over the analysis period.
Germany is Expected to Hold a Significant Share in the Europe Vascular Access Device Over the Forecast Period
- Germany is expected to witness significant growth over the forecast period owing to factors such as cardiovascular diseases, a vast geriatric population, a high number of chemotherapies, and growing awareness regarding vascular access devices among patient populations.
- For instance, according to data published by Germany Trade and Invest by the Federal Ministry of Economic Affairs and Climate Action, in 2022, over 24.0 million people will be 65 years and older (approximately 31% of the total population) by 2035. Since the elderly population is more vulnerable to several lifestyle disorders, the demand for surgical treatments is anticipated to grow over the coming years. Moreover, the same source further detailed that long-term diseases and chronic conditions account for 80% of German healthcare spending. Such factors are likely to augment the adoption of treatments and surgeries for medical ailments, ultimately boosting the country's demand for vascular access devices.
- Furthermore, according to the Robert Koch Institute 2021 report, cardiovascular diseases are the leading cause of death in Germany, causing a total of approximately 40% of all deaths. The demand for surgical procedures is likely to surge shortly to combat the overall fatalities caused by CVDs within the country. Since the delivery of fluids, blood products, medications, and other treatments to the bloodstream for patients with CVDs is made possible via central venous access devices (CVADs) or central venous catheters (CVCs), the demand for such devices is anticipated to grow.
- Also, the recent developments by the market players are expected to increase the penetration of vascular access devices in Germany, which will bolster the market growth in the country. For instance, in September 2022, Bentley, a Germany-based medical device company, acquired the rights to the GoBack catheter from Upstream Peripheral Technologies. The acquisition will enable the company to manufacture vascular access devices such as peripheral catheters in Germany by 2025.
- Therefore, owing to the high burden of chronic diseases, the vast geriatric population, and the recent developments by the market players, the market in Germany is anticipated to grow over the coming years.
Europe Vascular Access Device Industry Overview
The Europe vascular access device market is moderately fragmented in nature due to the presence of several companies operating globally as well as regionally. The major players operating in the market include Smiths Medical, Inc., B.Braun Melsungen Ag, Prodimed, Becton, Dickinson and Company, and Teleflex Incorporated.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Smiths Medical, Inc.
- B.Braun Melsungen Ag
- Prodimed
- Becton, Dickinson and Company
- Teleflex Incorporated
- Edwards Lifesciences Corporation
- AngioDynamics
- Terumo Medical Corporation
- 3M
- Nipro Medical Corporation
- Baxter International Inc.
- Fresenius Medical Care AG & Co. KGaA