Speak directly to the analyst to clarify any post sales queries you may have.
Establishing the contemporary context for women's health and beauty supplements with product, regulatory, and consumer dynamics reshaping commercial strategies
The women's health and beauty supplements sector is evolving at multiple intersecting vectors as consumers demand efficacy, transparency, and convenience from ingestible products that promise visible benefits. Shifts in ingredient science, distribution mechanics, and regulatory expectations have converged to make product differentiation as much about clinical credibility and traceability as it is about sensory experience and brand narrative.Over recent years, consumer behavior has migrated toward solutions that combine beauty and wellness, creating an expanded mental shelf where supplements support skin, hair, nails, and systemic health concurrently. Consequently, product developers and commercial teams must reconcile functional claims with evidence standards while delivering formats that match modern lifestyles. Reformulation, label clarity, and claims substantiation now represent operational priorities that intersect R&D, regulatory affairs, and marketing.
Transitioning from concept to commercialization requires an integrated lens: supply chain partners must guarantee ingredient provenance, manufacturing partners must accommodate novel formats such as gummies and plant-based proteins, and channels must be calibrated to reach segmented age cohorts. This introduction establishes the context for deeper analysis across market dynamics, tariff impacts, segmentation strategies, regional behaviors, and recommended actions for industry leaders.
How ingredient innovation, format evolution, and direct-to-consumer channels are reshaping the competitive dynamics and commercialization approaches in the sector
The landscape for women's health and beauty supplements has undergone transformative shifts driven by converging trends in science, consumer expectations, and retail innovation. Ingredient innovation, particularly in nutraceuticals such as collagen technologies, targeted probiotics, and adaptogenic herbal extracts, has moved beyond novelty into mainstream development, prompting more brands to invest in clinical validation and transparent sourcing practices.Concurrently, format innovation has redefined convenience and adherence. Consumers increasingly prefer formats that integrate into daily routines, favoring powder and gummy formats for ease of use alongside traditional capsules and tablets. Meanwhile, the rise of plant-based protein formulations and a stronger focus on bioavailability have forced manufacturers to reassess delivery systems and excipient strategies. Digital commerce and direct-to-consumer engagement have accelerated personalization, enabling brands to gather consumer data that informs iterative product development and targeted messaging.
As a result, the competitive field now rewards brands that can combine ingredient credibility with sensory appeal and omnichannel distribution. Industry stakeholders must therefore prioritize cross-functional collaboration to translate scientific advancements into consumer-facing benefits while maintaining regulatory compliance and supply chain resilience.
Assessing the operational and strategic consequences of United States tariff shifts on ingredient sourcing, formulation strategies, and distribution economics through 2025
The cumulative impact of recent tariff activity originating from the United States has introduced structural pressures across procurement, pricing strategy, and supply chain design for supplement manufacturers and distributors. Tariffs have affected input costs for botanicals, specialty extracts, and raw ingredient concentrates that often cross borders multiple times during processing. As a result, procurement teams have accelerated supplier diversification and regional sourcing strategies to mitigate exposure to tariff volatility and customs inspection delays.These pressures have translated into several operational responses. Manufacturers have increased inventory buffers for critical ingredients while investing in dual-sourcing arrangements to preserve continuity. Reformulation has become more prevalent where ingredient cost changes materially affect product economics, prompting R&D teams to evaluate functionally equivalent alternatives and reformulate with an eye toward preserving efficacy and sensory characteristics. In parallel, some companies have restructured their pricing architecture to absorb a portion of cost escalation while protecting brand value, and others have revised pack configurations to maintain unit margins without degrading perceived value.
From a trade compliance perspective, importers have tightened tariff classification and origin documentation to minimize dispute risk and reduce audit exposure. These adjustments have encouraged longer-term shifts, including greater investment in domestic manufacturing capacity, nearshoring of certain production stages, and closer collaboration with logistics partners to optimize landed cost. Consequently, industry players that proactively adapt sourcing, formulation, and logistics strategies will better manage the cumulative effects of tariff policy changes while maintaining competitiveness in both offline and online channels.
Strategic segmentation insights that connect product formulations, formats, demographics, packaging choices, distribution channels, and application-driven development
Key segmentation insights reveal how product development, packaging, and channel strategy must align with consumer expectations across multiple dimensions. Based on product type, industry participants are navigating demand across amino acids and derivatives, antioxidants, collagen and protein supplements, herbal and natural extracts, minerals, prebiotics and probiotics, and vitamins, with collagen and protein offerings further differentiated by plant-based and whey protein approaches that address distinct consumer values related to sustainability and performance. Based on form, decision-makers must weigh tradeoffs between capsules, gummies, liquids, powder, softgels, and tablets when optimizing for convenience, stability, and perceived efficacy.Age-based segmentation shows nuanced adoption patterns: adults, middle-aged consumers, older women, and young adults each bring distinct priorities for formulation, dosing, and claims language, which requires age-appropriate communications and package formats. Packaging type decisions-whether blister packs, bottles, pouches, or sachets and stick packs-impact preservation of actives, dosing accuracy, and on-shelf storytelling. Distribution channel strategy remains bifurcated between offline and online pathways; the offline channel encompasses health practitioner and clinic partnerships, pharmacies and drugstores, specialty health and beauty stores, and supermarkets and hypermarkets, all of which demand tailored merchandising and educational approaches. Finally, application focus between beauty and health drives SKU design: beauty applications segment into hair care, nail care, and skin care, while health applications cover bone and joint support, energy and metabolism, hormonal health, immune support, iron and anemia assistance, stress and sleep management, urinary and vaginal health, and weight management, which collectively dictate ingredient combinations and evidence requirements.
Regional behavioral, regulatory, and retail distinctions that demand localized product strategies across Americas, Europe, Middle East & Africa, and Asia-Pacific
Regional insights demonstrate that geographic nuance determines consumer preferences, supply chain decisions, and regulatory posture across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, consumers prioritize convenience and clinically positioned products, and the region features strong direct-to-consumer channels and pharmacy-led trust models that reward transparent claims and certifications. Meanwhile, Europe, Middle East & Africa presents a mosaic of regulatory regimes and cultural preferences where localization of claims, language, and ingredient approval pathways becomes essential for market entry and sustained growth.Asia-Pacific remains notable for rapid innovation adoption, strong demand for beauty-from-within propositions, and a complex ecosystem of cross-border trade that influences ingredient availability and cost. Manufacturers targeting Asia-Pacific often prioritize localized formulations and format variants suited to regional taste and consumption rituals. Across regions, differences in retail mix, reimbursement landscapes, and regulatory expectations require tailored go-to-market strategies. Consequently, organizations must integrate regional intelligence into product roadmaps, compliance planning, and channel selection to achieve resonance with local consumer cohorts while preserving scalable operational models.
Competitive dynamics and partnership models that define market leaders, emerging digital brands, and contract manufacturers in women's health and beauty supplements
Competitive insights reveal a blended ecosystem of legacy supplement manufacturers, emerging direct-to-consumer brands, ingredient innovators, and contract manufacturing organizations. Market leaders emphasize vertical integration or preferred supplier relationships to secure exclusive ingredient access and to control quality across complex value chains. At the same time, digitally native brands have leveraged data-driven marketing and community engagement to accelerate trial and build trust, often partnering with science-driven ingredient suppliers to substantiate claims and differentiate formulations.Strategic partnerships and collaborations are increasingly common, as companies seek to combine clinical expertise with scalable manufacturing and robust distribution networks. Private label and white-label manufacturers continue to enable retail and pharmacy chains to expand their assortments rapidly, while specialized contract developers provide formulation and regulatory support for novel delivery systems, such as enhanced-bioavailability complexes and multi-action capsules. The competitive landscape therefore prizes agility in product development, speed to market, and the ability to demonstrate safety and efficacy through credible third-party testing or published studies, creating opportunities for firms that can operationalize scientific rigor alongside compelling consumer storytelling.
Practical and prioritized strategic steps for companies to fortify sourcing, substantiate claims, optimize portfolios, and accelerate channel-specific growth
Actionable recommendations for industry leaders focus on aligning commercial priorities with operational capabilities to capture long-term value. First, strengthen ingredient sourcing strategies by diversifying supplier bases and developing validated secondary sources to reduce tariff and logistics vulnerabilities while pursuing certified traceability where feasible. Second, prioritize evidence generation for high-impact SKUs through targeted clinical studies, consumer usage trials, and third-party analytical testing to support substantiated claims and to enhance trade relationships with pharmacies and health practitioners.In addition, optimize portfolio architecture by segmenting SKUs by age cohort, application, and channel preference, and by rationalizing pack formats to balance unit economics with convenience and sustainability goals. Invest in format innovation that improves adherence, such as palatable gummies, fast-dissolving powders, and plant-based protein systems, and align packaging choices with both preservation needs and retail requirements. Finally, develop a regulatory intelligence function to monitor tariff developments, labeling changes, and cross-border compliance, and pair that with digital capabilities that enable rapid iteration of marketing messages and personalized consumer engagement to strengthen lifetime value and retention.
A rigorous mixed-methods research approach combining primary interviews, product audits, consumer analytics, and regulatory review to validate strategic insights
The research methodology combines qualitative and quantitative approaches to build a robust evidence base for strategic decision-making. Primary research included in-depth interviews with industry executives across R&D, supply chain, regulatory affairs, and commercial functions, along with structured discussions with retail buyers and health practitioners to capture on-the-ground perspectives about product performance, merchandising preferences, and consumer education needs. Supplementing these interviews, product audits and ingredient provenance reviews were conducted to assess manufacturing practices, packaging integrity, and labeling consistency across representative SKUs.Quantitative inputs were derived from anonymized point-of-sale trends, online consumer behavior analytics, and proprietary survey data designed to test claim credibility and format preferences across defined age cohorts. The methodology also incorporated a regulatory review to map approval pathways, labeling requirements, and tariff classifications relevant to cross-border trade. Data synthesis emphasized triangulation across sources to ensure that insights reflect operational realities and stakeholder priorities, while sensitivity checks and expert validation rounds reinforced the credibility of strategic recommendations.
Synthesis of strategic priorities that link clinical credibility, supply chain resilience, and regional go-to-market execution for sustained competitive advantage
In conclusion, the women's health and beauty supplements arena is characterized by accelerating innovation, evolving consumer expectations, and a more complex global trade environment that requires integrated responses across R&D, sourcing, and commercial operations. Brands that invest in clinical validation, transparent supply chains, and format convenience will resonate more strongly with consumers across age cohorts, while the ability to adapt to tariff-driven cost dynamics will separate resilient operators from those exposed to margin compression.Moreover, regional nuance and channel-specific demands necessitate localized strategies that preserve global scalability. By aligning portfolio architecture with consumer applications and by operationalizing regulatory intelligence, industry players can navigate short-term disruptions while building the foundations for sustainable growth. The synthesis of segmentation insights, regional behaviors, and competitive dynamics presented here equips leaders to make informed strategic choices that prioritize efficacy, compliance, and consumer relevance.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Women Health & Beauty Supplements Market
Companies Mentioned
The key companies profiled in this Women Health & Beauty Supplements market report include:- Abbott Laboratories
- Amway Corp. by Alticor
- ATP Science
- Bayer AG
- Blackmores Limited. by Kirin Holdings Co.
- Designs for Health, Inc.
- ELCA Cosmetics Pvt. Ltd.
- Fairhaven Health, LLC by Blueroot Health
- Glanbia PLC
- GNC Holdings, Inc. by Harbin Pharmaceutical Group
- Grape King Bio Ltd.
- Gynoveda Femtech Private Limited
- Herbalife Nutrition Ltd.
- Herbs of Gold Pty Ltd by Vita Life Sciences Ltd.
- Himalaya Global Holdings Ltd.
- Jarrow Formulas, Inc.
- L’Oréal S.A.
- Nature’s Care Manufacture Pty Ltd.
- Nestlé S.A
- NOW Health Group, Inc.
- Nu Skin Enterprises, Inc.
- Pfizer, Inc.
- Pharmavite LLC by Otsuka Pharmaceutical Co., Ltd.
- Procter & Gamble Limited
- Suntory Holdings Limited
- Swisse Wellness Pty Ltd. by H&H Group
- Taisho Pharmaceutical Holdings Co., Ltd.
- Unilever PLC
- Usana Health Sciences, Inc.
- Zeroharm Sciences Private Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2025 - 2030 |
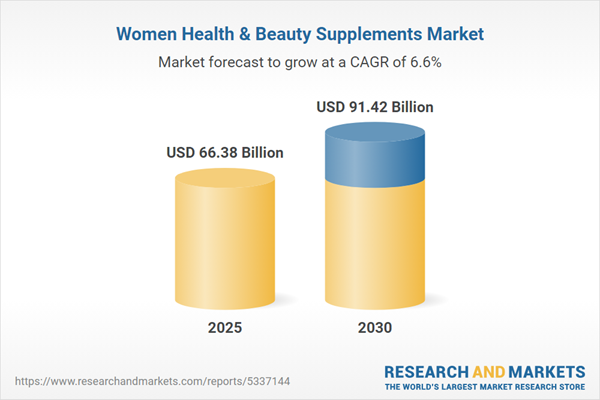
| Estimated Market Value ( USD | $ 66.38 Billion |
| Forecasted Market Value ( USD | $ 91.42 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 31 |