Peptide therapeutics are polypeptides or peptides (oligomers or short polymers of amino acids) that are utilized to treat disorders. Peptide treatments replicate natural peptide functions such as hormones, neurotransmitters, growth factors, ion channel ligands, and anti-infectives. Because peptides may be digested by the body, they are thought to be reasonably safe and well-tolerated.
Liraglutide, the most popular diabetic medicine on the market, contains a lipid chain that increases plasma circulation and provides long-term absorption. Liraglutide is a GLP-1 agonist that self-assembles itself into an alpha-helical structure and only needs to be consumed once a day. Liraglutide's half-life in the blood is lengthened by approximately 13-14 hours due to lipid conjugation of a palmitoyl chain to a lysine residue at position 26. This is attributed to the palmitoyl chain's ability to bind albumin non-covalently, delaying DPP IV's proteolytic action and enabling fast renal clearance. The inclusion of the lipid chain could further extend the half-life by preventing the DPP IV enzyme from degrading sterically.
Lanreotide, an octapeptide, is another peptide that may self-assemble. This medication is a synthetic counterpart of the peptide hormone somatostatin, which is used to alleviate acromegaly (a malfunction where the body produced excessive amounts of growth hormone). Lanreotide assembles itself into monodisperse liquid crystalline nanotubes when exposed to water. The nanotubes are composed of dimers that self-assemble into a 2D lattice that is kept together by antiparallel ß-sheets and lateral chain connections. Studies on self-assembling amyloid aggregates created by peptide hormones and neuropeptides have provided more insight into how self-assembly, as well as peptide hormones, are connected.
COVID-19 Impact Analysis
COVID-19 was addressed using novel peptides that have been repurposed as medicines. Researchers throughout the world are still looking for chemicals that can either stop SARS-CoV-2 infection and replication or relieve symptoms caused by the virus. The FDA revised its definition of a biologic to include chemically produced polypeptides with a length of more than 40 amino acids but less than 100 amino acids (synthetic proteins) and synthetic peptides with a length of 40 amino acids or less (synthetic peptides). There were 21 peptide medicines in development for the treatment of COVID-19, including 15 synthetic peptides in therapies for the treatment of ARDS and other respiratory disorders caused by SARS-Cov-2 infection.Market Growth Factors
Rising number of applications
Cardiovascular disease (CVD) is still the major cause of death and morbidity around the world. Many interventions are being tested to ameliorate pathological cardiovascular problems, however, there have been few new drugs licensed for intervention or treatment. As a result, novel techniques for treating CVD are urgently needed. The treatment of contributory risk factors and underlying mechanisms, such as inflammation, obesity, hyperglycemia, and hypercholesterolemia is frequently used to prevent vascular problems. The pharmaceutical industry has traditionally resisted using peptides as therapeutic agents due to their limited stability, size, rate of breakdown, and poor distribution. However, there has been a renaissance in the development of peptides and their synthetic derivatives for therapeutic intervention in recent years.Increasing cases of cancer all over the world
Cancer is the second leading cause of death, accounting for a considerable number of deaths all over the world in 2013. The rate of cancer in developed countries is significantly higher than in developing countries. The prevalence of known risk factors is predicted to rise in these under-developed nations due to population growth and aging, as well as an increased prevalence of known risk factors. Lung cancer is the largest cause of death from cancer in men around the world, but only in industrialized countries, while breast cancer is the leading reason of cancer death in developing countries. Somatic gene mutations that change the function of the proteins they encode have been linked to cancer. Most solid tumors, like those of the brain, colon, breast, and pancreas, exhibit somatic changes.Market Restraining Factors
Side effects and risks related to peptide therapy
Natural polypeptides like hormones, growth factors, and neurotransmitters, unlike synthesized peptide medications, are considered to play an important function in normal physiology. Membrane impermeability and in vivo instability are two key disadvantages of peptide medicines. Proteolytic degradation of peptide medicines in serum diminishes the bioavailable concentration and reduces the drug's half-life. To keep the medicine at a clinically effective concentration, routine dosing may be required. To avoid proteolytic degradation and enhance the in vivo half-life of peptide medicines, a variety of chemical modification approaches have been used.Type Outlook
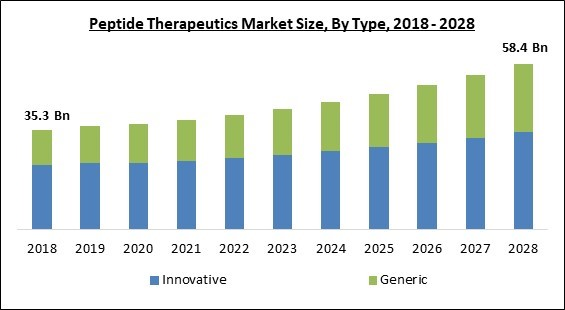
Based on Type, the market is segmented into Innovative and Generic. In 2021, the innovative segment procured the highest revenue share of the peptide therapeutics market. The constantly rising growth of the segment is owing to significant R&D investments by major pharmaceutical companies in the development of newer pharmaceuticals and high prescription rates. Based on kind, the market is segmented into generic and inventive categories.Route of Administration Outlook
Based on Route of Administration, the market is segmented into Parenteral Route, Pulmonary, Mucosal, Oral Route, and Others. In 2021, the pulmonary segment registered a significant revenue share of the peptide therapeutics market. The pulmonary route is recognized for peptide and protein delivery because of the physiological properties of the respiratory system. Numerous proteins and peptides are now being tested in clinical studies and are seeking authorization for use in the lungs.Synthesis Technology Outlook
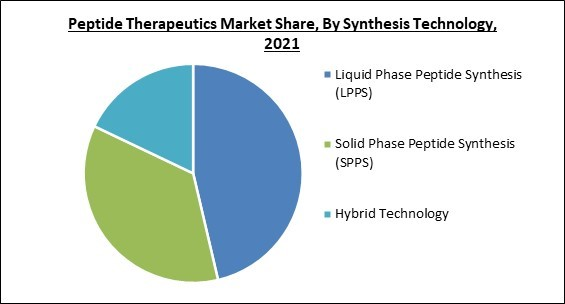
Based on Synthesis Technology, the market is segmented into Liquid Phase Peptide Synthesis (LPPS), Solid Phase Peptide Synthesis (SPPS), and Hybrid Technology. In 2021, the Liquid Phase Peptide Synthesis segment recorded the highest revenue share of the peptide therapeutics market. The expediting growth of the segment is owing to the increased need for pure peptides for the development of effective medicines. Consumer preferences are evolving to quicker and more accurate approaches such as LPPS as a result of challenges such as greater time consumption. Hence, the growth of the segment is flourishing.Type of Manufacturers Outlook
Based on Type of Manufacturers, the market is segmented into In-house and Outsourced. In 2021, the outsourcing segment recorded a significant revenue share of the peptide therapeutics market. The growth of the segment is rising due to the challenges in developing therapeutics that meet high-quality standards. Businesses choose to outsource the Active Pharmaceutical Ingredient (API) to companies with advanced technology and expertise in the production of peptides of various sorts.Application Outlook
Based on Application, the market is segmented into Metabolic, Cardiovascular Disorder, GIT & Renal, Antiinfection & Dermatology, Respiratory, Central Nervous System, Cancer, Pain, and Others. In 2021, the metabolic segment acquired the largest revenue share of the peptide therapeutics market. The increasing growth of the segment is attributed to the fact that metabolic problems are becoming increasingly common. These problems have become more common as a result of sedentism, bad eating habits, and excessive alcohol usage. Furthermore, the market is likely to benefit from the growing geriatric population, which is sensitive to metabolic problems.Cardinal Matrix-Peptide Therapeutics Market Competition Analysis
Regional Outlook
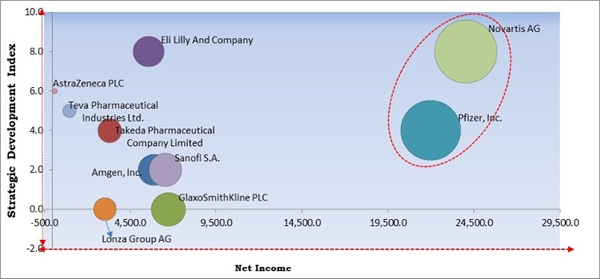
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. In 2021, North America accounted for the highest revenue share of the peptide therapeutics market. The growing awareness of peptide treatments, rising demand for cancer and other disease diagnostics, and the burgeoning biotechnology industry are all contributing to the growth of the regional market. Peptide therapies are also likely to benefit from growing government R&D spending throughout the coming years. The well-established pharmaceutical and biopharmaceutical industry in this region is a primary driver of growth.The major strategies followed by the market participants are Partnerships and Approvals. Based on the Analysis presented in the Cardinal matrix; Pfizer, Inc. and Novartis AG are the forerunner in the Peptide Therapeutics Market. Companies such as Eli Lilly And Company, Sanofi S.A. and Takeda Pharmaceutical Company Limited are some of the key innovators in the Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Eli Lilly And Company, AstraZeneca PLC, Teva Pharmaceuticals Industries Ltd., Novartis AG, Pfizer, Inc., Sanofi S.A., GlaxoSmithKline PLC, Takeda Pharmaceutical Company Limited, Lonza Group AG, and Amgen, Inc.
Strategies Deployed in Peptide Therapeutics Market
Partnerships, Collaborations and Agreements:
- Mar-2022: Sanofi partnered with Adagene, a platform-driven, clinical-stage biopharmaceutical company. Under this partnership, the companies is expected to manufacture and commercialize masked monoclonal and bispecific antibodies in order to introduce transformative novel medicines to cancer patients all over the world.
- Jan-2022: Amgen joined hands with Generate Biomedicinesm, a therapeutics company. Under this collaboration, the companies is expected to focus on the discovery and development of protein therapy for five clinical targets within certain therapeutic areas as well as multiple modalities.
- Dec-2021: Eli Lilly and Company came into a multi-year research collaboration and licensing agreement with Regor Therapeutics Group, a clinical-stage company. Under this agreement, the companies is expected to discover, manufacture, and commercialize new therapies to cure metabolic disorders. Moreover, Regor's technology is expected to also enable Eli Lilly to drive innovation and offer breakthrough therapies for diabetes and obesity.
- Jul-2021: Takeda Pharmaceutical Company teamed up with PeptiDream, a biopharmaceutical company. Following this partnership, the companies is expected to develop peptide-drug conjugates for certain central nervous system targets, which cause chronic neurodegenerative diseases.
- Jul-2020: AstraZeneca entered into an agreement with Daiichi Sankyo Company, an innovative global healthcare company. Following this agreement, the companies aimed to expand the reach of the DS-1062 to more patients all over the world in a shorter period of time.
Product Launches and Product Expansions:
- Apr-2020: Teva Pharmaceuticals released the autoinjector device for AJOVY. With this launch, the company aimed to offer preventive treatment for migraine to adult patients. Moreover, the new product is the only anti-CGRP preventive migraine treatment.
Acquisitions and Mergers:
- May-2022: Pfizer took over Biohaven Pharmaceutical, a commercial-stage biopharmaceutical company. Following this acquisition, the company aimed to incorporate Biohaven's Rimegepant and Zavegepant along with an offering of five pre-clinical CGRP assets.
- Jul-2021: Eli Lilly and Company completed its acquisition of Protomer Technologies, a biotech company. With this acquisition, the company aimed to integrate the glucose-sensing insulin program of Protomer Technologies into its portfolio in order to strengthen its offerings. In addition, Eli Lilly is expected to enhance the diabetes lineup of Protomer with the company's innovative technology
- Sep-2020: AstraZeneca acquired the preclinical oral PCSK9 inhibitor program of Dogma Therapeutics, an operator of a biotechnology platform. Following this acquisition, the company aimed to advance the program into the clinical development for dyslipidemia and familial hypercholesterolemia.
Approvals and Trails:
- May-2022: Eli Lilly and Company received the US FDA approval for its Mounjaro injection. The novel once-weekly GIP and GLP-1 receptors agonist are approved as an adjunct to exercise and diet in order to enhance glycemic control within adults with type 2 diabetes.
- Mar-2022: Novartis received the US FDA approval for its Pluvicto. Through this approval, the company aimed to offer a treatment to adult patients with prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer, a type of advanced cancer. In addition, Pluvicto is the first radioligand therapy approved by the US FDA that incorporates a therapeutic radioisotope and a targeting compound.
- Sep-2021: Teva Pharmaceuticals and MedinCell received the US FDA approval for their TV-46000/mdc-IRM. The new product is approved on the basis of the RISE Study and SHINE Study, two Phase III clinical trials, for the treatment of schizophrenia.
Scope of the Study
Market Segments Covered in the Report:
By Type
- Innovative
- Generic
- Parenteral Route
- Pulmonary
- Mucosal
- Oral Route
- Others
- Liquid Phase Peptide Synthesis (LPPS)
- Solid Phase Peptide Synthesis (SPPS)
- Hybrid Technology
- In-house
- Outsourced
- Metabolic
- Cardiovascular Disorder
- GIT & Renal
- Antiinfection & Dermatology
- Respiratory
- Central Nervous System
- Cancer
- Pain
- Others
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Eli Lilly And Company
- AstraZeneca PLC
- Teva Pharmaceuticals Industries Ltd.
- Novartis AG
- Pfizer, Inc.
- Sanofi S.A.
- GlaxoSmithKline PLC
- Takeda Pharmaceutical Company Limited
- Lonza Group AG
- Amgen, Inc.
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Eli Lilly And Company
- AstraZeneca PLC
- Teva Pharmaceuticals Industries Ltd.
- Novartis AG
- Pfizer, Inc.
- Sanofi S.A.
- GlaxoSmithKline PLC
- Takeda Pharmaceutical Company Limited
- Lonza Group AG
- Amgen, Inc.