Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Rising Burden of Chronic and Infectious Diseases
The increasing prevalence of chronic and infectious diseases is a major driver of the Saudi Arabia IVD market. Chronic diseases such as diabetes, hypertension, and cardiovascular conditions are on the rise due to changing lifestyles and dietary habits. Approximately 14.8% of men and 11.7% of women in Saudi Arabia live with diabetes, while hypertension affects 17.7% of men and 12.5% of women. The nation also has one of the highest rates of obesity and diabetes in the world, which directly correlates with a heightened demand for diagnostic monitoring tools.Cancer incidence is also increasing, with an age-standardized rate of 96.5 cases per 100,000 population in 2020. Two-thirds of all cancer cases in GCC countries are reported in Saudi Arabia. These health trends necessitate routine, long-term diagnostic testing for disease detection and monitoring, boosting the use of IVD products such as reagents, analyzers, and molecular testing kits. The expansion of diagnostic infrastructure and public health campaigns is further supporting the growing adoption of IVD technologies.
Key Market Challenges
Regulatory Complexity and Lengthy Approval Processes
Navigating the regulatory environment in Saudi Arabia remains a significant challenge for IVD manufacturers. While the Saudi Food and Drug Authority (SFDA) has made progress in establishing medical device regulations, compliance with stringent documentation, testing, and certification protocols is required before products can enter the market. Regulatory delays, frequent updates, and localization requirements - such as language adaptations and collaboration with local distributors - can extend time-to-market and increase operational costs. These obstacles are particularly burdensome for small and mid-sized companies, discouraging innovation and slowing the introduction of cutting-edge diagnostic technologies. Addressing these challenges through clearer regulatory guidelines and streamlined approval processes will be essential for unlocking the full potential of the IVD market in the region.Key Market Trends
Expansion of AI and Big Data in Healthcare
The integration of Artificial Intelligence (AI) and Big Data is emerging as a transformative trend in the Saudi Arabia IVD market. AI-driven diagnostic tools are being utilized in radiology, pathology, and laboratory workflows to enhance clinical accuracy, reduce turnaround times, and support early disease detection. Machine learning algorithms improve the sensitivity and specificity of diagnostics for conditions like cancer, cardiovascular disease, and diabetic complications. Big Data analytics enables the development of predictive models that can forecast patient outcomes, monitor disease progression, and tailor treatment strategies. Hospitals are leveraging AI to automate appointment scheduling, triage, and administrative processes, improving overall efficiency. As healthcare providers adopt these technologies, AI and Big Data are poised to enhance diagnostic precision, reduce medical errors, and deliver more personalized care - strengthening the role of IVD in Saudi Arabia’s digital health transformation.Key Market Players
- Altona Diagnostics GmbH
- Beckman Coulter, Inc.
- Siemens Healthineers AG
- OncoDNA SA
- Vela Diagnostics (Nile Science Corporation)
- Abbott Laboratories, Inc.
- Becton, Dickinson, and Company
- F. Hoffmann-La Roche AG
- Thermo Fischer Scientific, Inc.
- Sysmex Corporation
Report Scope:
In this report, the Saudi Arabia In Vitro Diagnostics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below.Saudi Arabia In Vitro Diagnostics Market, By Product:
- Instruments
- Reagent
- Software & Services
Saudi Arabia In Vitro Diagnostics Market, By Technology:
- Immunoassay
- Hematology
- Clinical Chemistry
- Molecular Diagnostic
- Microbiology
- Others
Saudi Arabia In Vitro Diagnostics Market, By Application:
- Infectious Diseases
- Diabetes
- Oncology
- Cardiology
- Nephrology
- Others
Saudi Arabia In Vitro Diagnostics Market, By End-User:
- Hospitals & Clinics
- Diagnostic Centers & Laboratories
- Others
Saudi Arabia In Vitro Diagnostics Market, By Region:
- Eastern
- Western
- Northern & Central
- Southern
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Saudi Arabia In Vitro Diagnostics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Altona Diagnostics GmbH
- Beckman Coulter, Inc.
- Siemens Healthineers AG
- OncoDNA SA
- Vela Diagnostics (Nile Science Corporation)
- Abbott Laboratories, Inc.
- Becton, Dickinson, and Company
- F. Hoffmann-La Roche AG
- Thermo Fischer Scientific, Inc.
- Sysmex Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 85 |
| Published | April 2025 |
| Forecast Period | 2024 - 2030 |
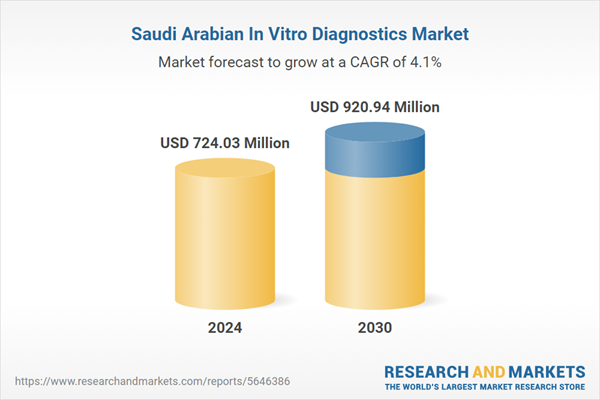
| Estimated Market Value ( USD | $ 724.03 Million |
| Forecasted Market Value ( USD | $ 920.94 Million |
| Compound Annual Growth Rate | 4.0% |
| Regions Covered | Saudi Arabia |
| No. of Companies Mentioned | 10 |