Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative overview describing how scientific innovation and commercial dynamics are jointly reshaping antibodies and reagents for research and therapeutics
The antibodies and reagents domain is a cornerstone of contemporary life sciences activity, powering discovery science, diagnostic innovation, and therapeutic development. Advances in antibody engineering, novel reagent chemistries, and assay technologies have converged to expand the technical toolbox available to researchers and clinicians. At the same time, commercial dynamics such as evolving sourcing strategies, regulatory expectations, and cross-border trade policies are reshaping how products are developed, manufactured, and delivered to end users across academia, industry, and contract research organizations.
This executive summary synthesizes the essential trends and strategic implications for stakeholders across the value chain. It provides context on scientific innovations that are altering product portfolios, reviews structural changes in supply and distribution that influence procurement decisions, and highlights segmentation and regional patterns that inform go-to-market choices. Throughout, the emphasis is on actionable interpretation-translating technical developments and policy shifts into clear implications for product planning, partnership formation, and operational resilience.
Readers should expect a focused, evidence-driven narrative that balances scientific nuance with commercial clarity. The objective is to equip research leaders, procurement heads, and senior executives with the insights needed to prioritize investments, adapt sourcing strategies, and align internal capabilities with the fastest-moving opportunities in antibodies and reagents.
A concise synthesis of converging scientific, operational, and commercial shifts that are accelerating innovation and raising quality expectations across antibodies and reagents
The antibodies and reagents landscape is undergoing a series of transformative shifts that are redefining competitive advantage, from technology platforms to business models. First, the maturation of engineered antibody formats and recombinant production methods has driven improvements in reproducibility and batch consistency, which in turn influences buyer confidence and downstream assay performance. Next, assay technologies such as high-sensitivity immunoassays and multiplexed workflows have expanded the functional use cases for both antibodies and a diverse array of reagents, encouraging integrated solutions that bundle detection chemistry with validated antibody pairs.
Concurrently, supply chain modernization and heightened quality standards are prompting manufacturers to revise supplier networks and invest in traceability systems. This shift favors firms that can demonstrate robust quality management, transparent sourcing, and rapid lot-to-lot comparability. Furthermore, the increasing adoption of digital engagement-ranging from e-commerce platforms for reagent procurement to cloud-based experiment documentation-has accelerated the integration of product data with laboratory informatics, enabling smoother procurement cycles and improved consumable utilization.
Finally, collaborative models between reagent developers and end users are becoming more prominent. Co-development partnerships, strategic licensing of novel antibody clones, and targeted reagent validation programs are all enabling faster translation of discoveries into validated assays. Taken together, these shifts are compressing the time from innovation to validated application and are rewarding organizations that combine scientific rigor with agile commercial execution.
A practical assessment of how recent United States tariff measures are reshaping sourcing, contractual practices, and supply resilience in the antibodies and reagents ecosystem
Recent tariff developments originating from the United States have introduced additional complexity into global sourcing decisions for antibodies and reagents, affecting cost structures, supplier selection, and inventory strategies. Tariff-driven changes in import duties and customs processing have influenced the relative attractiveness of different manufacturing locations, prompting both suppliers and purchasers to reassess the geography of raw material procurement and finished goods distribution. As a result, some manufacturers have sought to diversify production footprints, while certain buyers have moved to increase safety stocks or to hold strategic buffer inventories to insulate critical projects from supply interruptions.
In addition, the indirect impacts of tariff regimes have manifested in contractual negotiation practices and supplier relationships. Longer-term supply agreements increasingly incorporate clauses related to tariff exposure, cost pass-through mechanisms, and contingency logistics. These contractual adaptations are intended to preserve continuity of supply while fairly allocating commercial risk between buyers and suppliers. At the operational level, expanded customs scrutiny and reclassification activity have required logistics teams to invest in enhanced tariff code management and in closer collaboration with customs brokers and legal advisors.
Importantly, the cumulative effect of tariff pressures has not only been cost-related but has accelerated strategic choices about localization, supplier qualification, and vertical integration. Organizations with flexible sourcing strategies and strong quality governance have been better positioned to respond to these pressures, while others have accelerated internal initiatives to validate alternate suppliers and to build redundancy into critical reagent supply chains. In sum, tariff dynamics have catalyzed a broader reassessment of resilience and commercial alignment across the sector.
Detailed segmentation analysis connecting product types, biological sources, assay technologies, disease priorities, application needs, and end-user expectations across the value chain
A granular view of segmentation illuminates where scientific demand and commercial opportunity intersect across product, source, technology, disease area, application, and end user. When considering product type, antibodies and reagents differentiate along primary, recombinant and secondary antibody lines and an extensive array of reagent classes such as buffers, cell culture reagents, cytokines, detection chemistries, enzymes, and nucleic acids. The distinctions between monoclonal and polyclonal primary antibodies and the continued rise of recombinant antibody formats influence customer preferences for specificity, reproducibility, and supply continuity. Reagents that support genomics and proteomics workflows are particularly important for advanced discovery applications, creating cross-functional buying patterns.
Source differentiation-mouse-derived, rabbit-derived, and rat-derived origins-remains a meaningful factor for affinity characteristics, immunogenicity considerations, and compatibility with assay systems, and influences both R&D selection and regulatory documentation. Technology segmentation underscores the centrality of techniques such as enzyme-linked immunosorbent assays, flow cytometry, immunofluorescence, immunohistochemistry, immunoprecipitation, lateral flow devices, mass spectrometry, and Western blotting; within immunoassays, the split between competitive and sandwich formats further refines product validation strategies and reagent pairings.
Disease area priorities such as cardiology, infectious disease, neurology, and oncology drive targeted reagent development where subdomains like atherosclerosis, hypertension, bacterial and viral infections, Alzheimer’s and Parkinson’s diseases, and breast and lung cancers necessitate specific antibody panels and validated reagents. Application-driven segmentation across diagnostics, drug development, research and development, and therapeutics-where R&D itself branches into genomics and proteomics-creates differential demand for high-throughput compatibility, regulatory-grade documentation, and lot-to-lot consistency. Finally, end users including academic research institutions, biopharmaceutical companies, and contract research organizations exhibit distinct procurement behaviors, with academic customers prioritizing breadth and cost-effectiveness, biopharma emphasizing GMP-readiness and supplier risk management, and CROs valuing scalability and validated supply chains.
An integrated regional perspective revealing how Americas, Europe-Middle East-Africa, and Asia-Pacific dynamics shape supply chains, regulatory needs, and commercial strategies
Regional dynamics exert a powerful influence on strategy, supply chain configuration, and commercial focus across the antibodies and reagents arena. In the Americas, the presence of leading academic institutions, biotechnology clusters, and a mature diagnostics industry drives strong demand for validated reagents and high-performance antibodies; this region also places emphasis on regulatory alignment and rapid access to novel research tools. Moving to Europe, the Middle East and Africa, there is a pronounced emphasis on harmonized quality standards and collaborative research networks, where cross-border partnerships and centralized manufacturing hubs support broad geographic coverage while meeting diverse regulatory regimes.
In the Asia-Pacific region, capacity expansion and an increasing number of local biomanufacturing capabilities are reshaping global sourcing. Rapidly growing R&D investment, scaling biopharma ecosystems, and the emergence of regional centers of excellence are increasing the demand for both standard reagents and advanced antibody formats. Each region exhibits distinct procurement rhythms and regulatory expectations, which in turn affect product registration timelines, validation requirements, and distribution models. Consequently, effective regional strategies balance localized supply capabilities with centralized quality oversight, enabling companies to serve heterogeneous demand while preserving consistent performance standards across global portfolios.
Across all regions, cross-border collaboration, investments in local manufacturing, and strategic alliances with regional distributors are central to achieving resilient and responsive supply chains. These regional patterns should inform decisions about where to invest in manufacturing capacity, how to design regulatory dossiers, and which commercial partnerships will most effectively translate R&D innovations into adopted laboratory practices.
A strategic assessment of company behaviors showing how technological leadership, manufacturing quality, and partnership models define competitive advantage
Competitive dynamics among leading companies in the antibodies and reagents space are driven by a combination of technological differentiation, manufacturing excellence, and customer-centric validation services. Top-performing organizations are investing heavily in recombinant technologies, high-affinity clone discovery, and reagent chemistries that enhance stability and assay sensitivity. In parallel, leading companies are strengthening their value proposition through expanded validation datasets, application notes, and cross-platform compatibility testing that reduce adoption friction for laboratory scientists.
Strategic partnerships and ecosystem plays have become more prominent as firms seek to complement in-house capabilities with specialized service providers. Collaborations that bundle antibodies with assay-ready reagents, or that provide co-developed panels for specific disease indications, are effective at shortening the path from discovery to validated application. Additionally, investments in manufacturing quality systems, cold-chain logistics, and lot traceability differentiate incumbents by reassuring regulated buyers and large biopharmaceutical accounts.
Commercially, companies that offer flexible procurement options, transparent lot release data, and responsive technical support are gaining preference among institutional purchasers. At the same time, a subset of firms is pursuing vertical integration to capture more control over raw material inputs and to accelerate time-to-availability for high-demand reagents. Going forward, leadership will favor organizations that combine deep scientific IP with operational resilience, strong data-driven validation, and service models that align closely with the workflows of academic, biopharma, and CRO customers.
Clear, actionable strategic priorities for executives to combine scientific investment, supply diversification, and customer-centric services to secure competitive leadership
Industry leaders should pursue a multi-faceted strategy that balances scientific innovation with operational resilience and commercial agility. First, prioritizing investment in recombinant antibody discovery and rigorous validation reduces downstream variability and enhances credibility with regulated buyers. Pairing these technical investments with application-focused reagent bundles and validated protocols can accelerate adoption in both discovery and diagnostic settings. Second, diversifying manufacturing footprints and qualifying alternate suppliers for critical raw materials will mitigate tariff and logistics exposures while supporting faster regional fulfillment.
Third, strengthening customer-facing capabilities-such as comprehensive lot documentation, interactive technical support, and seamless integration with laboratory informatics-will differentiate suppliers in a crowded field. In addition, leaders should pursue strategic partnerships with academic centers and biopharma innovators to co-develop reagent suites targeted at high-priority disease areas; these collaborations can create defensible pipelines and new validation datasets. Fourth, companies should incorporate flexible commercial terms and transparent contractual mechanisms that fairly allocate trade-related costs and provide customers with predictable procurement pathways during regulatory or trade disruptions.
Finally, committing resources to sustainability, ethical sourcing, and supply chain transparency will increasingly matter to institutional customers. By aligning R&D priorities with operational investments and customer-centric services, organizations can both capture near-term opportunities and build resilient foundations for long-term growth in the antibodies and reagents ecosystem.
A transparent, rigorously validated research approach combining expert interviews, literature synthesis, and cross-validated segmentation mapping to ensure actionable insights
The research methodology underpinning this analysis combines qualitative expert engagement and rigorous data triangulation to produce actionable insights that reflect both scientific realities and commercial dynamics. Primary research included structured interviews with senior scientific leaders, procurement executives, and supply chain specialists to capture first-hand perspectives on adoption drivers, validation requirements, and sourcing constraints. These conversations were complemented by a systematic review of peer-reviewed literature, regulatory guidance, product technical files, and publicly available corporate disclosures to ground qualitative findings in documented evidence.
Data synthesis relied on cross-validation across independent sources to ensure reliability. Segmentation frameworks were mapped to product catalogs, assay applications, and end-user workflows to capture the nuanced ways in which reagent types and antibody formats are used in different research and clinical contexts. Regional analyses incorporated regulatory frameworks and manufacturing footprints to explain geographic differences in procurement and validation practices. Throughout the methodology, quality controls included iterative analyst reviews, reconciliation of divergent viewpoints, and the use of reproducible documentation practices to support traceability of findings.
This methodological approach emphasizes transparency, reproducibility, and context-sensitive interpretation so that stakeholders can assess not only what the data indicate but also how to apply the insights to operational and strategic decisions within their organizations.
A compelling synthesis stressing the need to integrate scientific rigor with operational foresight to sustain research continuity and competitive advantage
In conclusion, the antibodies and reagents landscape is at a pivotal juncture where scientific advancement, supply chain discipline, and commercial ingenuity intersect to determine competitive outcomes. Technical innovations-particularly in recombinant antibody engineering and assay integration-are expanding the scope of what laboratory teams can achieve, while external pressures such as tariff dynamics and regional manufacturing shifts are reshaping procurement and operational choices. Taken together, these forces increase the value of suppliers who can deliver validated, reproducible materials alongside strong quality systems and responsive service models.
For stakeholders across academia, biopharma, and contract research, the imperative is to align procurement, R&D, and supply chain strategies with evolving assay demands and regional realities. Executives should prioritize supplier relationships that demonstrate technical depth, transparent quality data, and the capacity to adapt to regulatory and trade fluctuations. By doing so, organizations will enhance their ability to advance discovery, accelerate translational pathways, and sustain reliable operations in the face of uncertainty.
Overall, the sector rewards those who combine scientific rigor with operational foresight, and the recommendations and insights contained in this summary are intended to help leaders make informed choices that strengthen both short-term responsiveness and long-term competitiveness.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
18. China Research Antibodies & Reagents Market
Companies Mentioned
The key companies profiled in this Research Antibodies & Reagents market report include:- Abbott Laboratories
- Agilent Technologies Inc.
- Alpha Teknova, Inc.
- Atlas Antibodies AB
- Avantor, Inc.
- BASF SE
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Bio-Techne Corporation
- bioMérieux S.A.
- Cardinal Health, Inc.
- Cell Signaling Technology, Inc.
- Charles River Laboratories International, Inc.
- Danaher Corporation
- Dovetail Genomics LLC
- Enzo Life Sciences, Inc.
- F. Hoffmann-La Roche Ltd.
- FUJIFILM Holdings Corporation
- GenScript Biotech Corporation
- Honeywell International Inc.
- Illumia, Inc.
- ImmunoPrecise Antibodies Ltd.
- Leinco Technologies, Inc.
- LifeSpan BioSciences, Inc.
- LobaChemie Pvt. Ltd.
- Lonza Group Ltd.
- Merck KGaA
- Omega Bio-tek, Inc.
- PerkinElmer, Inc.
- QIAGEN N.V.
- Randox Laboratories Ltd.
- Rockland Immunochemicals, Inc.
- Santa Cruz Biotechnology, Inc.
- Sino Biological, Inc.
- Thermo Fisher Scientific, Inc.
- Tokyo Chemical Industry Co., Ltd.
- Tosoh Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | January 2026 |
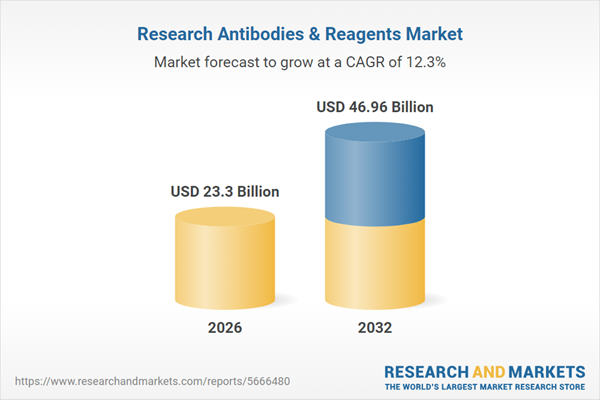
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 23.3 Billion |
| Forecasted Market Value ( USD | $ 46.96 Billion |
| Compound Annual Growth Rate | 12.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 38 |