Speak directly to the analyst to clarify any post sales queries you may have.
An authoritative introduction detailing the clinical profile of Darier disease, therapeutic challenges, evolving treatment paradigms, and unmet patient needs
Darier disease, a rare genodermatosis with protean clinical manifestations, presents a persistent therapeutic challenge that intersects dermatology, genetics, and chronic care management. Patients typically endure chronic papular eruptions, malodor, and nail changes, all of which combine to produce substantial morbidity and quality-of-life impairment. Clinicians and clinical development teams confront a heterogeneous phenotype that complicates standardized treatment algorithms and requires nuanced therapeutic approaches that balance efficacy, tolerability, and long-term safety.
Recent clinical practice emphasizes symptom control through topical measures, selective systemic therapy for severe cases, and multidisciplinary care models that incorporate dermatologists, genetic counselors, and nursing staff. Pharmacologic interventions frequently adapt agents from related dermatologic or inflammatory indications, while academic centers explore targeted molecular strategies informed by advances in calcium signaling and keratinocyte biology. As translational science progresses, the therapeutic landscape is trending toward precision interventions that address underlying pathogenic pathways rather than only symptomatic lesions. This introductory section orients stakeholders to the disease’s burden, current therapeutic philosophies, and the translational opportunities that influence clinical decision-making and drug development priorities.
Rapid shifts in therapeutic development and trial design are accelerating innovation, regulatory alignment, and repositioning of Darier disease therapies
Developmental momentum in the Darier disease therapeutic arena is increasingly shaped by shifts in clinical trial design, translational biology insights, and regulatory pathways that incentivize innovation for rare dermatologic disorders. Adaptive trial designs, broader use of real-world evidence, and more flexible endpoints are enabling sponsors to evaluate novel molecules and repurposed agents with greater efficiency, especially where classic large randomized trials are impractical. Concurrently, deeper mechanistic understanding of ATP2A2 dysfunction and downstream keratinocyte signaling has opened actionable targets that were previously theoretical, allowing biopharma teams to prioritize candidates with plausible disease-modifying potential.
The regulatory environment is also evolving to better accommodate rare-disease programs through expedited review modalities and more receptive dialogues on surrogate endpoints, which collectively lower barriers for first-in-class therapies. Industry alliances and academic consortia are accelerating translational pipelines by pooling phenotypic datasets and harmonizing outcome measures, thereby reducing duplication and elevating evidence quality. Taken together, these transformative shifts are recalibrating how therapeutic value is defined for Darier disease, moving the field from symptomatic management toward interventions with durable impact on clinical progression and patient-centered outcomes.
Operational and commercial repercussions from tariff changes are compelling supply chain resilience, sourcing diversification, and distribution redesign for specialty dermatology products
The introduction of enhanced trade measures and tariff adjustments in 2025 has required pharmaceutical stakeholders to re-evaluate global supply chain architectures for specialty dermatology products. Active pharmaceutical ingredient sourcing, excipient procurement, and finished-dose manufacturing have felt pressure as duties and customs complexity increased for certain cross-border flows. Manufacturers and suppliers have responded by intensifying supplier qualification efforts, diversifying material sources, and engaging in more robust scenario planning to preserve continuity for critical production runs. These operational responses frequently include renegotiating long-term supply contracts and identifying tariff classification strategies that legally minimize duty exposure.
From a distribution perspective, increased import costs have led logistics teams to reassess inventory deployment, prioritizing buffer stocking at regional distribution centers and selectively relocating manufacturing steps closer to key demand centers. Payers and hospitals have grown more attentive to cost-per-treatment drivers, prompting tighter formulary negotiations and more rigorous utilization review for high-cost specialty agents. To mitigate distribution friction, some firms have expanded direct-to-clinic and direct-to-patient fulfillment capabilities, while others have accelerated parallel import management and tariff-compliant labeling alternatives. In aggregate, the 2025 tariff environment has sharpened commercial discipline and reinforced the strategic importance of resilient procurement, regional manufacturing optimization, and transparent cost epidemiology for Darier disease therapeutics.
Integrated segmentation insights linking drug class, administration route, distribution channels, end users, and product typologies to clinical and commercial dynamics
A granular view of product segmentation provides clarity on how therapeutic options for Darier disease are organized and evaluated across development and commercial pathways. Based on Drug Class, the landscape is studied across Anti Infectives, Corticosteroids, and Retinoids, with Anti Infectives further analyzed across Oral Antibiotics and Topical Antibiotics, Corticosteroids further differentiating Systemic Corticosteroids and Topical Corticosteroids, and Retinoids separated into Systemic Retinoids and Topical Retinoids, reflecting both symptomatic and disease-modifying strategies. This drug-class perspective helps stakeholders map clinical use cases to formulation choices and safety considerations, particularly where systemic exposure is a concern.
Based on Route Of Administration, therapeutic options are studied across Oral, Parenteral, and Topical, with Oral formulations further divided into Capsules and Tablets, Parenteral represented by Injections, and Topical split among Creams, Gels, and Ointments, which underscores the operational differences in adherence, tolerability, and compounding needs. Based on Distribution Channel, the portfolio is examined across Hospital Pharmacies, Online Pharmacies, and Retail Pharmacies, with Hospital Pharmacies further categorized into Private Hospital Pharmacies and Public Hospital Pharmacies, Online Pharmacies dissected into Ecommerce Platforms and Pharmacy Websites, and Retail Pharmacies sub-segmented into Chain Pharmacies and Independent Pharmacies, highlighting commercial access routes and channel-specific reimbursement dynamics.
Based on End User, therapeutic delivery is framed across Dermatology Clinics, Home Care Settings, and Hospitals, with Dermatology Clinics further delineated into Multi Specialty Clinics and Specialized Clinics, Home Care Settings broken out into Nursing Care and Self Administration, and Hospitals differentiated into Private Hospitals and Public Hospitals, which together illuminate clinical adoption patterns and training requirements. Based on Product Type, offerings are classified into Branded and Generic, with Branded segmented into First Generation and Next Generation and Generic distinguishing First Entry Generics and Multi Source Generics, demonstrating the lifecycle progression from innovation to broader accessibility and the competitive forces that shape price and availability.
Regional clinical, regulatory, and supply chain dynamics shaping access, evidence requirements, and manufacturing strategies across global geographies
Regional imperatives exert strong influence on clinical practice patterns, regulatory strategy, and supply chain execution for Darier disease therapeutics. In the Americas, clinical care pathways emphasize integrated dermatology networks and specialty pharmacies that support chronic management programs, while regulatory pathways provide mechanisms for accelerated review in rare disease contexts and private sector payer negotiations shape access dynamics. In Europe, Middle East & Africa, heterogeneity in regulatory frameworks and reimbursement environments creates variable adoption rates, with centralized European agencies and national health technology assessment bodies exerting significant influence on availability and on evidence requirements for novel therapeutics.
Across Asia-Pacific, regulatory modernization, expanding specialist care capacity, and growing local manufacturing capabilities have combined to create a rapidly evolving environment for product registration and distribution. Regional supply chains are adapting to local content policies and trade agreements, prompting firms to evaluate regional manufacturing hubs and contract development and manufacturing organization partnerships. Cross-region collaboration on clinical registries and consensus outcome measures is increasingly common, enabling comparative effectiveness evaluations that inform regional formulary decisions and guide clinical guideline updates across diverse health systems.
Corporate strategies, partnership models, and manufacturing partnerships that determine clinical progress, commercialization readiness, and competitive differentiation in rare dermatology
Key company behavior in the Darier disease space reveals an interplay between innovation-focused biotechs, specialty dermatology units within larger pharmaceutical firms, and contract manufacturers supporting scalable supply. Innovator companies are concentrating on mechanism-driven assets and strategic partnerships with academic centers to accelerate translational programs, while specialty units within larger firms are leveraging clinical development expertise and global regulatory experience to de-risk late-stage programs. Contract manufacturing and specialty formulation providers are increasingly critical partners, enabling rapid scale-up for topical and systemic formulations and offering clinical supply chain management essential for orphan indications.
Commercial strategies emphasize lifecycle management via next-generation formulations, label expansions, and combination approaches that pair symptomatic agents with adjunctive therapies. Licensing arrangements and co-development agreements remain common as firms seek to share development risk and leverage complementary capabilities. Importantly, patient advocacy groups and clinical centers of excellence are playing a more visible role in shaping trial recruitment, natural history studies, and real-world evidence initiatives, which in turn influences company prioritization and resource allocation across portfolios. These dynamics collectively create an ecosystem where nimble R&D, reliable manufacturing partnerships, and stakeholder engagement determine competitive positioning.
Practical, high-impact actions for stakeholders to align translational research, adaptive development, and resilient commercialization for durable therapeutic success
Industry leaders seeking to advance therapeutic solutions for Darier disease should prioritize an integrated strategy that links translational research, pragmatic clinical development, and resilient commercialization pathways. First, invest in targeted translational programs that leverage genetic and cellular disease models to validate candidate mechanisms and identify biomarkers that can meaningfully stratify responders. Second, adopt adaptive and decentralized clinical trial models that reduce patient burden and accelerate evidence generation while maintaining rigorous endpoint validation. Third, strengthen supply chain resilience by diversifying API sources, qualifying secondary suppliers, and considering regional manufacturing footprints to mitigate tariff and logistics disruptions.
Commercial actions should include early payer engagement and the development of comprehensive value dossiers that emphasize patient-centered outcomes and long-term safety. Develop strategic collaborations with dermatology centers and patient advocacy organizations to enhance trial recruitment, natural history data collection, and dissemination of best practices. Additionally, consider tiered product strategies that combine branded innovation with planned generic access pathways to maximize both short-term clinical impact and long-term affordability. Finally, embed real-world evidence frameworks into post-approval plans to capture longitudinal outcomes, support label refinement, and inform continuous product optimization.
Robust mixed-methods research design combining expert interviews, source triangulation, and iterative validation to ensure credible and actionable insights
The research approach underpinning this analysis integrates qualitative and quantitative methods to ensure robustness and relevance for stakeholders. Primary research included structured interviews with clinical experts, specialty pharmacists, and supply chain managers, which provided insights into clinical practice variability, formulation preferences, and operational constraints. Secondary sources were synthesized to contextualize recent scientific advances, regulatory guidance, and standard-of-care practice patterns, and all findings were triangulated to resolve discrepancies and strengthen interpretive confidence.
Analytical rigor was maintained through iterative expert validation rounds, in which draft conclusions and hypotheses were reviewed and refined based on practitioner feedback. Data quality controls included cross-referencing regulatory documents, clinical trial registries, and peer-reviewed literature to confirm clinical and operational assertions. Limitations of the methodology are acknowledged, including potential geographic bias in primary interview representation and the inherent lag between emerging scientific developments and published confirmation. To mitigate these factors, the methodology emphasizes transparent documentation of sources and an ongoing update cadence for high-priority sections.
Concluding synthesis highlighting translational momentum, operational imperatives, and strategic levers required to convert clinical promise into durable therapeutic impact
In closing, the therapeutic landscape for Darier disease is at an inflection point where mechanistic insights, adaptive clinical development approaches, and pragmatic commercial strategies converge to create new opportunities for patients and stakeholders. Clinical care is gradually evolving from predominantly symptomatic management toward approaches that incorporate targeted interventions and personalized application of existing agents. Supply chain and trade dynamics add complexity but also incentivize more resilient sourcing and regional execution strategies that can preserve continuity of care.
For decision-makers, the combination of scientific momentum and changing regulatory receptivity presents a compelling environment to prioritize investments in biomarker-driven programs, flexible trial architectures, and partnerships that amplify distribution capabilities. The path forward requires coordinated action across R&D, regulatory affairs, commercial operations, and patient engagement functions to translate scientific advances into meaningful clinical improvements and sustainable access models. This synthesis highlights the critical levers that will determine whether emerging therapies achieve durable clinical impact and equitable patient access.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Darier Disease Drugs Market
Companies Mentioned
The key companies profiled in this Darier Disease Drugs market report include:- AbbVie Inc
- Amneal Pharmaceuticals Inc
- Bausch Health Companies Inc
- BridgeBio Pharma Inc
- Galderma SA
- GlaxoSmithKline plc
- Glenmark Pharmaceuticals Ltd
- Johnson & Johnson
- Mayne Pharma Group Ltd
- Merck & Co Inc
- Novartis AG
- Pfizer Inc
- Sigmapharm Laboratories LLC
- Sun Pharmaceutical Industries Ltd
- Teva Pharmaceutical Industries Ltd
- Tolmar Pharmaceuticals Inc
- Validus Pharmaceuticals LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 198 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
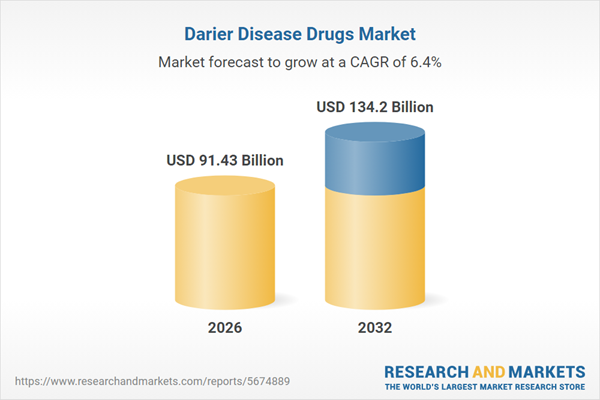
| Estimated Market Value ( USD | $ 91.43 Billion |
| Forecasted Market Value ( USD | $ 134.2 Billion |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |