Speak directly to the analyst to clarify any post sales queries you may have.
Introduction to the evolving patient needs, formulation innovation, and omnichannel access reshaping commercial strategies for allergy relieving eye drops
Allergic conjunctivitis remains a persistent public health concern, driven by environmental changes, urbanization, and evolving seasonal exposures, and allergy relieving eye drops sit at the intersection of clinical need and consumer convenience. Patients are increasingly empowered to self-manage ocular allergy symptoms, and this behavior has elevated the importance of accessible, efficacious formulations that provide fast relief with minimal side effects. As a result, pharmaceutical development and commercial strategies have shifted toward optimizing user experience, ensuring regulatory compliance, and enabling omnichannel availability.
Beyond therapeutic performance, formulation innovation has become a differentiator; companies now balance multi-mechanism approaches with tolerability profiles that support repeated use. Concurrently, healthcare stakeholders are placing greater emphasis on evidence generation, adherence support, and real-world outcomes that demonstrate sustained symptom control. Supply chains and channel partners are adapting to changing procurement patterns, while payers and clinicians are recalibrating recommendations to reflect both safety considerations and patient preference for over-the-counter access. Taken together, these forces underscore a dynamic landscape where clinical effectiveness, consumer-centric design, and strategic distribution converge to shape the future of allergy relieving eye drops.
Transformative clinical advances, digital adoption, and evolving retail dynamics converging to redefine competitive strategies in the ocular allergy therapeutic space
The landscape for allergy relieving eye drops is undergoing transformative shifts driven by converging clinical, commercial, and technological trends that are redefining competitive advantage. Advances in pharmaceutical science have enabled combination therapies and optimized delivery systems that extend duration of action while minimizing irritation, prompting incumbent manufacturers to revisit product roadmaps and launch cadence. At the same time, the rise of digital health tools and telemedicine has changed how consumers discover, evaluate, and obtain ocular therapies, leading to closer integration between clinical advice and retail access.
Consumer behavior has shifted toward convenience and transparency, increasing demand for clear labeling, evidence of safety, and accessible guidance on appropriate use. Retail dynamics are evolving in parallel: online retail growth is prompting manufacturers to invest in direct-to-consumer channels as well as third-party partnerships, while traditional pharmacy formats continue to evolve with differentiated services. Regulatory frameworks are also adapting to scientific innovation and OTC transitions, creating both opportunities and compliance imperatives. In sum, strategic winners will be those who can harmonize product innovation, digital engagement, and regulatory agility to meet rapidly changing expectations.
Cumulative effects of 2025 trade policy adjustments on supply chain resilience, cost-to-serve dynamics, and procurement strategies in ocular allergy therapeutics
Recent tariff changes and trade policy adjustments in the United States during 2025 have exerted cumulative effects across pharma supply chains, logistics planning, and cost structures relevant to allergy relieving eye drops. Import duties and compliance requirements on excipients, primary packaging, and finished goods have elevated landed costs for products and raw materials sourced from affected jurisdictions. In response, manufacturers have explored a range of mitigation strategies, from reshoring key components to diversifying supplier networks and renegotiating long-term contracts to stabilize input pricing and lead times.
These policy shifts have also influenced inventory management and procurement rhythms; firms have increased buffer inventories for critical components while accelerating validation of alternate suppliers to reduce exposure to single-source risks. At the commercial interface, pricing strategy and promotional planning have been adjusted to preserve margin while maintaining accessibility, accompanied by more rigorous cost-to-serve analyses across distribution channels. Moreover, regulatory compliance and customs clearance complexities have driven investment in trade advisory services and supply chain transparency solutions. Moving forward, proactive scenario planning and flexible manufacturing partnerships will be essential to absorb further policy gyrations and to protect continuity of care for patients.
Segment-driven strategies that connect end-user pathways, channel architecture, formulation types, and product forms to inform prioritized commercialization choices
A granular understanding of segmentation dynamics is essential for shaping targeted product development and channel strategies. Based on End User, differentiation between Over The Counter and Prescription pathways dictates regulatory requirements, labeling expectations, and the nature of clinical evidence needed to support each route; OTC positioning prioritizes ease of use and safety for repeated consumer application, while prescription products emphasize clinician-directed efficacy and may support more complex combination approaches. Based on Distribution Channel, the mix of Convenience Stores, Hospital Pharmacy, Online Retail, and Pharmacy Stores influences assortment, merchandising, and promotional tactics; within Online Retail, distinct behaviors on Company Owned Website versus Third Party Marketplace shape customer experience design and control over pricing, whereas Pharmacy Stores split into Chain Pharmacy and Independent Pharmacy channels each require tailored trade engagement and stocking strategies.
Ingredient Type segmentation-Antihistamine, Combination, Decongestant, and Mast Cell Stabilizer-frames formulation strategy and messaging, with antihistamines addressing immediate symptom relief, mast cell stabilizers supporting preventative control, combination therapies offering synergistic benefits, and decongestants presenting tolerability trade-offs. Application segmentation across Perennial and Seasonal use cases informs lifecycle management and promotional rhythms, with perennial offerings needing sustained adherence support and seasonal products requiring surge-ready distribution. Finally, Form segmentation of Multi Dose and Single Dose affects packaging design, patient convenience, sterility considerations, and environmental footprint decisions. Integrating these segmentation lenses provides a multidimensional view for prioritizing R&D investment, channel activation, and commercial messaging.
How regional regulatory nuances, consumer behaviors, and distribution ecosystems across the Americas, Europe Middle East & Africa, and Asia-Pacific shape differentiated market approaches
Regional dynamics present differentiated opportunities and operational considerations that should inform go-to-market sequencing and resource allocation. In the Americas, regulatory frameworks and high consumer propensity for over-the-counter self-care create fertile ground for retail-led expansion and digital engagement, while reimbursement landscapes and payer scrutiny require careful positioning of prescription alternatives. Europe, Middle East & Africa display varied regulatory regimes and diverse purchasing behaviors; Western European markets often demand rigorous clinical evidence and premium positioning, whereas emerging economies within the region prioritize affordability and supply chain robustness, necessitating flexible packaging and distribution models. Asia-Pacific reflects rapid digital adoption and heterogeneous regulatory pathways across jurisdictions, presenting opportunities for rapid online scale and for partnerships that localize formulation and packaging to meet diverse climatic and usage patterns.
These regional distinctions carry implications for manufacturing footprint decisions, regulatory dossier strategies, and commercial partnerships. Companies must align clinical evidence generation, pricing architecture, and channel mixes to regional preferences while preserving global brand coherence. Cross-regional learning can accelerate adoption; for example, digital patient support tools piloted in highly connected markets can be adapted to enhance adherence in regions with different healthcare access profiles. Ultimately, a regionally nuanced approach balances standardization and localization to capture durable demand.
Competitive dynamics driven by formulation innovation, strategic partnerships, and channel specialization that determine brand differentiation and operational resilience
Competitive dynamics in the allergy relieving eye drops arena are shaped by a combination of legacy brands, innovative entrants, and contract manufacturers that enable rapid scale. Leading companies are investing in next-generation formulations, evidence generation programs, and brand differentiation strategies that emphasize tolerability, onset of action, and duration of relief. Vertical integration of key manufacturing steps and strategic partnerships with packaging and distribution specialists are increasingly common as firms seek to secure capacity and manage cost volatility. Smaller, agile players are leveraging digital channels and direct-to-consumer engagement to build niche followings, while larger firms deploy their scale to support global launches and extensive promotional reach.
In addition, collaboration between pharmaceutical developers and clinical research organizations is accelerating the translation of mechanistic advances into clinically meaningful endpoints, supporting both prescription and OTC claims. Supply-side partners, including contract manufacturing organizations and ingredient suppliers, play a pivotal role in enabling formulation flexibility and speed-to-market. The interplay between brand equity, clinical credibility, operational resilience, and channel execution will determine relative competitiveness, with patient trust and clinician endorsement remaining critical differentiators in a crowded landscape.
Actionable strategic imperatives to strengthen supply resilience, validate product differentiation, and harmonize omnichannel execution for sustainable growth
Industry leaders should adopt a pragmatic set of actions to strengthen resilience, accelerate innovation, and capture differentiated demand in allergy relieving eye drops. First, prioritize formulation portfolios that balance rapid onset with long-term tolerability, supported by robust real-world evidence and targeted clinical studies to validate claims across both OTC and prescription pathways. Second, diversify supply chains and establish multiple validated suppliers for critical excipients and packaging components, while investing in inventory optimization and scenario-based contingency planning to mitigate policy-driven disruptions.
Third, advance omnichannel commercialization by harmonizing direct-to-consumer platforms with pharmacy partnerships and hospital channels, ensuring consistent messaging and patient support across Company Owned Website, Third Party Marketplace, Chain Pharmacy, and Independent Pharmacy environments. Fourth, tailor regional strategies to align with distinct regulatory expectations and consumer preferences across the Americas, Europe, Middle East & Africa, and Asia-Pacific, leveraging digital tools where appropriate to scale education and adherence programs. Finally, embed sustainability and patient-centric design into product lifecycle decisions, optimizing packaging formats such as single dose and multi dose to address sterility, convenience, and environmental concerns. Taken together, these actions will enable organizations to convert insight into measurable competitive advantage.
A multi-source research methodology combining primary stakeholder interviews, clinical and regulatory synthesis, and supply chain analysis to produce actionable industry intelligence
This research approach integrates multiple evidence streams to produce a coherent, actionable analysis of the allergy relieving eye drops landscape. Primary intelligence is gathered through structured interviews with clinicians, pharmacists, channel leaders, and industry executives to capture frontline perspectives on prescribing behavior, consumer preferences, and operational constraints. Secondary research synthesizes peer-reviewed clinical literature, regulatory guidance, patent filings, and publicly available regulatory agency communications to contextualize product claims and compliance requirements. Additionally, supply chain mapping and trade flow analysis inform assessment of vulnerability points and logistics considerations.
Analytical methods include qualitative thematic analysis of stakeholder interviews, comparative assessment of formulation attributes, and scenario planning to evaluate the implications of trade policy changes and distribution shifts. Cross-validation routines ensure congruence between primary insights and secondary evidence while expert review panels provide critical appraisal of assumptions and interpretations. The outcome is a pragmatic intelligence package that prioritizes actionable implications and supports decision-making across R&D, commercial, regulatory, and supply chain functions.
Synthesis of clinical, operational, and commercial imperatives that inform strategic priorities and enable resilient decision-making in the ocular allergy category
Allergy relieving eye drops occupy a dynamic intersection of clinical necessity, consumer self-care, and evolving commercial channels, demanding integrated strategies that address formulation, distribution, and evidence generation. The cumulative effects of trade policy shifts, digital retail expansion, and formulation innovation require companies to be both nimble and deliberate: nimble in diversifying suppliers and adjusting channel execution, and deliberate in investing in clinically credible differentiation and patient support. Regional variability underscores the need for localized regulatory strategies and tailored go-to-market plans, while segmentation insights highlight the importance of aligning product form and ingredient choice to end-user needs and channel expectations.
Looking ahead, companies that combine rigorous clinical validation, resilient supply operations, and sophisticated omnichannel engagement will be best positioned to meet patient needs and sustain commercial momentum. Cross-functional alignment, supported by scenario-driven planning and targeted investment, will convert market complexity into strategic clarity and operational advantage. In this environment, timely access to integrated intelligence and consultative support will accelerate decision-making and help stakeholders prioritize initiatives with the highest potential impact.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Allergy Relieving Eye Drops Market
Companies Mentioned
The key companies profiled in this Allergy Relieving Eye Drops market report include:- Abbott Laboratories
- AbbVie Inc.
- Alcon Inc.
- Alembic Pharmaceuticals Limited
- Astellas Pharma Inc.
- Bausch Health Companies Inc.
- Bayer AG
- Dr. Reddy’s Laboratories Ltd.
- Hoffmann-La Roche Ltd.
- Johnson & Johnson
- Lupin Limited
- Merck & Co., Inc.
- Nicox S.A.
- Novartis AG
- Ocular Therapeutix, Inc.
- Pfizer Inc.
- Prestige Consumer Healthcare Inc.
- ROHTO Pharmaceutical Co., Ltd.
- Rynel Clifton Pharma Pvt. Ltd.
- Sager Pharma Kft.
- Santen Pharmaceutical Co., Ltd.
- Similasan Corporation
- Starpharma Holdings Limited
- Sumitomo Pharma Co., Ltd.
- Sun Pharmaceutical Industries Limited
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
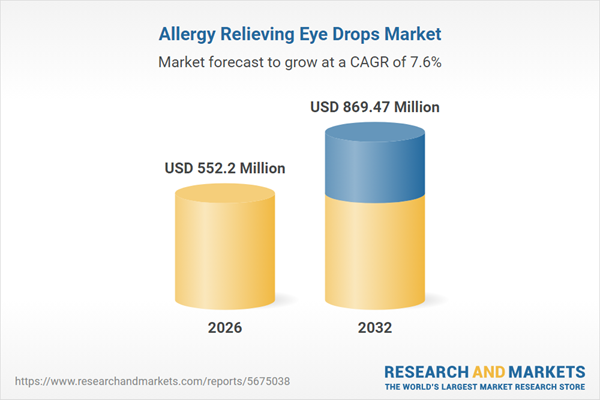
| Estimated Market Value ( USD | $ 552.2 Million |
| Forecasted Market Value ( USD | $ 869.47 Million |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |