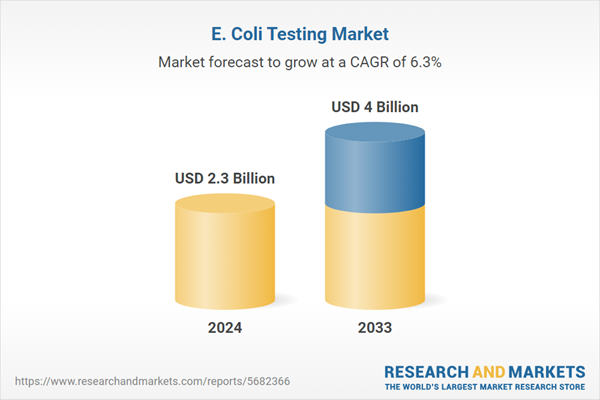
The global E. coli testing market is witnessing expansion chiefly due to heightening awareness regarding water and foodborne illnesses, combined with stricter regulatory frameworks for water and food safety. Amplifying incidents of E. coli outbreaks have incentivized accelerated need for innovative testing methods in emerging as well as developed regions. In addition to this, technological innovations in molecular diagnostics and the bolstering utilization of automated testing systems are further fueling market growth. Moreover, the growing emphasis on public sanitation and health, specifically in urban zones, is prompting heavy investments in advanced testing infrastructure, magnifying the adoption of E. coli testing solutions worldwide.
The United States accounts for a crucial share in the global E. coli testing market, majorly propelled by stringent implementation of food safety policies, resilient healthcare infrastructure, and amplifying public awareness associated with foodborne illnesses. The heightening occurrence of E. coli outbreaks in the food and beverage sector has significantly strengthened the requirement for dependable testing solutions. For instance, in November 2024, Grimmway Farms, a prominent U.S.-based carrot producer, recalled organic baby carrots due to E. coli outbreak, resulting in 39 cases across 18 U.S. states. In addition, elevating investments in research projects and advancements in rapid testing technologies further aid in market expansion. Furthermore, with regulatory bodies, encompassing the USDA and FDA enforcing stringent adherence, several businesses are rapidly opting for leading-edge diagnostic tools to address risks and facilitate customer safety. This resilient regulatory landscape supports constant market growth.
E. oli Testing Market Trends:
Growing Demand for Rapid Diagnostic Tests
Increases in the demand for quicker diagnostics in gastrointestinal infections, with the pathogen E. coli among the most relevant, have led towards high-speed testing methodologies. While classical laboratory culture takes two weeks for identification of the organism E. coli, newer PCR-based tests, such as Thermo Fisher's TaqPath Enteric Bacterial Select Panel, yield results within approximately two hours. This rapid turnaround allows health providers to diagnose and treat infections more promptly, which consequently decreases patient wait times and hospital stays. For instance, according to a research article, the TaqPath panel provides sensitivity and specificity of over 98%, ensuring that the results are always dependable. The trending towards fast diagnostic methods has been crucial in controlling foodborne disease outbreaks by being able to intervene in a timely manner to reduce the spread and effect of infections. It also allows for testing 93 samples in one run, which can improve laboratory throughput and increase the possibility of rapid diagnosis even with high-volume clinical settings.Increasing Focus on Food Safety Regulations
Stringent food safety regulations globally are propelling the demand for advanced E. coli testing solutions. Regulatory bodies are emphasizing compliance with testing protocols to mitigate risks of contamination in food production and distribution chains. For instance, as per industry reports, E. coli contamination causes approximately 73,000 illnesses each year in the U.S., resulting in around 2,200 hospitalizations and 61 fatalities. Moreover, this trend is driving the adoption of automated testing systems that deliver reliable and reproducible results, ensuring compliance while optimizing operational efficiency. In addition, rising consumer awareness regarding food safety and hygiene is further encouraging industries to invest in robust testing frameworks to safeguard public health and maintain brand integrity.Expansion of Point-of-Care Testing
Point-of-care (POC) testing for E. coli increasingly gains support through molecular diagnostic technologies like PCR, LAMP, and mass spectrometry, which provide rapid, on-site results for pathogens in water, food, and stool samples. PCR, particularly, is requisite for the detection of E. coli from water and stool samples, providing a more reliable method for healthcare providers to diagnose infections and initiate treatment in real time. The LAMP DNA amplification technique can also sense the presence of E. coli in food, stool and environmental samples in one step using a process that could be suited for field applications as well as resource-limited settings. According to a research article, the use of mass spectrometry is also realized in the typing of bacteria and viruses, such as E. coli, to study the subtypes and sources of infection in detail. IMS (immunomagnetic separation) facilitates the increase in the yield of recovery of E. coli O157:H7 from environmental water samples essential for outbreak studies. Other emerging technologies, including CRISPR/Cas platforms and smartphone-based digital methods, improve the accessibility and speed of E. coli detection, further pushing the adoption of molecular diagnostics in POC settings. These innovations contribute to more efficient and timely diagnosis, reducing healthcare costs and improving patient outcomes, particularly in urgent care and remote areas.E. Coli Testing Industry Segmentation:
The publisher provides an analysis of the key trends in each segment of the global E. coli testing market, along with forecast at the global, regional, and country levels from 2025-2033. The market has been categorized based on product, test type, and end user.Analysis by Product:
- Consumables
- Instruments
Analysis by Test Type:
- Environmental Test
- Membrane Filtration (MF)
- Multiple Tube Fermentation (MTF)
- Enzyme Substrate Methods
- Clinical Test
- Polymerase Chain Reaction (PCR) Tests
- Enzyme Immunoassays (EIA)
- Others
Analysis by End User:
- Hospitals and Clinics
- Diagnostic Laboratories
- Others
Regional Analysis:
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
Key Regional Takeaways:
United States E. Coli Testing Market Analysis
In 2024, United States accounted for the 87.60% of the market share in North America. E. coli infections are an important public health concern in the United States because it causes around 265,000 cases and 100 deaths per year. According to a research article, outbreaks and serious illnesses most often occur by Shiga toxin-producing E. coli, or about 40% of all infections from STEC are due to E. coli O157:H7. It shows the symptoms of infection in cases, including diarrhea, abdominal cramps, nausea, vomiting, and low-grade fever; its complications may be kidney failure or HUS. Young children, the elderly, and others who may be suffering from other illnesses fall within those at a higher risk of complications. E. coli further causes UTIs in up to 80% to 90% cases; this is especially seen among women. Recent outbreaks in the U.S. have included contaminated organic carrots and a severe outbreak linked to McDonald's Quarter Pounders in Mountain West states. Due to that, the demand for good E. coli testing in food safety, healthcare, and water treatment continues to grow.Europe E. Coli Testing Market Analysis
The E. coli testing market in Europe is growing because of strict food safety regulations and increasing health care issues. According to the European Centre for Disease Prevention and Control, in 2023, the EU had the incidence of third-generation cephalosporin-resistant E. coli bloodstream infections at 10.35 per 100,000 people, which is a problem that is increasing with respect to antimicrobial resistance. The ECDC also reported that E. coli isolates harbouring the blaNDM-5 gene were associated with travel abroad from the EU/EEA to Africa and Asia countries. STEC continues to remain a risk, and a recommendation has been made through ECDC to maintain correct hand hygiene, food preparation, and washing of the vegetables. E. coli continues to be a prominent cause of bacterial infections related to enteritis, urinary tract infections, septicaemia, and neonatal meningitis. The above factors increase the demand for high-tech diagnostic tools. Moreover, the countries driving the most demand and innovation are Germany, France, and the UK. Furthermore, food traceability and sustainability have gained focus, leading to investment in novel testing technologies, hence supporting the growth of this market.Asia Pacific E. Coli Testing Market Analysis
Asia Pacific E. coli testing market is growing at a higher rate due to increased food production and awareness of food safety. According to China's National Health Commission, rapid urbanization and industrial agriculture increase foodborne illnesses including E. coli infections. India reported more than 500 outbreaks of diarrheal diseases in 2023. Among the patient samples, the most common bacteria isolated were E. coli. This means that the governments and private sectors are focusing more on installing efficient testing systems, especially for food and beverage companies. Multiplex PCR and lateral flow immunoassays are popular emerging technologies. In addition, states like Japan and South Korea are actively introducing the most advanced testing processes for food exports. Furthermore, India is increasingly improving the testing infrastructure for domestic purposes. Asia Pacific is considered to be the key region for E. coli testing market growth due to continuously increasing regulatory pressure.Latin America E. Coli Testing Market Analysis
Rising food safety concerns and increasing outbreaks of foodborne diseases in Latin America is anticipated to increase the demand for E. coli testing. According to a 2023 industrial report, Brazil is a leading country in Latin America in food production and exports, which fuels the E. coli testing market. The meta-analysis of 4,286 samples found that the general prevalence rate of Shiga toxin-producing E. coli (STEC) in Brazil was 1%, with the highest rate in Mato Grosso at 9%. Hot carcasses had the highest rate of positive samples for STEC at 8%. The Ministry of Health in Brazil has stressed the need for testing in the face of food safety requirements, whether for local consumption or for export. Argentina and Mexico are also investing more in food safety and diagnostics. Furthermore, as awareness of foodborne pathogens increases, the region experiences a shift toward faster, more automated testing solutions. Moreover, harsher regulations and standards instituted by governments also complement demand for advanced testing technologies in the market. Regional cooperation furthers the market growth process, as local manufacturers find partnerships with global players to enhance testing capabilities.Middle East and Africa E. Coli Testing Market Analysis
The Middle East and Africa E. coli testing market is expected to grow due to increasing health issues and the increasing regulatory demands for food safety. According to the World Health Organization, foodborne diseases, including E. coli infection, are among the most concerning in the region and rise annually. The health ministry of Saudi Arabia in 2020 reported 1,270 cases of foodborne diseases, prompting the government to enforce stronger food safety regulations. Even in South Africa, where home-based producers export agricultural produce around the world, E. coli testing becomes important to reach international standards for food safety. Additionally, there is an expansion of both public and private investments in testing infrastructure within the region. Furthermore, the region's diverse geography as well as infrastructure challenges support widespread adoption of emerging technologies especially portable and rapid test kits.Competitive Landscape:
The market is highly competitive, with leading players focusing on innovation and strategic partnerships to gain market share. Major companies are actively investing in the development of advanced rapid testing solutions to meet the growing demand for efficient and accurate diagnostics. Furthermore, the market features fierce competition among established as well as emerging firms providing niche solutions. In addition, competitive differentiation is typically bolstered by adherence to regulatory policies, technological innovations, and cost-efficiency. Moreover, several companies are augmenting their geographic reach and improving product lines to address the evolving demands of a broader customer base, further magnifying competition in this dynamic market. For instance, in July 2024, Alden, a biotechnology firm, launched its new food safety and quality testing method Suspended Simultaneous Sandwich Assay. This method can efficiently detect E. coli O157 in beef products.The report provides a comprehensive analysis of the competitive landscape in the E. coli testing market with detailed profiles of all major companies, including:
- Accugen Laboratories Inc.
- Alere Inc. (Abbott Laboratories)
- BD (Becton Dickinson and Company)
- bioMérieux (INSTITUT MERIEUX)
- Bio-RAD Laboratories Inc.
- Enzo Life Sciences Inc. (Enzo Biochem Inc.)
- Idexx Laboratories Inc.
- Johnson & Johnson
- Meridian Bioscience Inc.
- Nanologix Inc.
- Pro-Lab Diagnostics
- Qiagen N.V.
- Thermo Fisher Scientific Inc.
Key Questions Answered in This Report
1. What is E. coli testing?2. How big is the E. coli testing market?
3. What is the expected growth rate of the global E. coli testing market during 2025-2033?
4. What are the key factors driving the global E. coli testing market?
5. What is the leading segment of the global E. coli testing market based on product?
6. What is the leading segment of the global E. coli testing market based on test type?
7. What is the leading segment of the global E. coli testing market based on end user?
8. What are the key regions in the global E. coli testing market?
9. Who are the key players/companies in the global E. coli testing market?
Table of Contents
Companies Mentioned
- Accugen Laboratories Inc.
- Alere Inc. (Abbott Laboratories)
- BD (Becton Dickinson and Company)
- bioMérieux (INSTITUT MERIEUX)
- Bio-RAD Laboratories Inc.
- Enzo Life Sciences Inc. (Enzo Biochem Inc.)
- Idexx Laboratories Inc.
- Johnson & Johnson
- Meridian Bioscience Inc.
- Nanologix Inc.
- Pro-Lab Diagnostics
- Qiagen N.V.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 147 |
| Published | August 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 2.3 Billion |
| Forecasted Market Value ( USD | $ 4 Billion |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |