For instance, toxicity, cytochrome p450 induction, and in vitro metabolism assays, among others, are being incorporated into a single assay on liver hepatocytes. Similarly, 3D cell culture models with multiple tissue cell types are emerging as promising systems.
Moreover, microfluidic organ-on-a-chip approaches, such as those commercialized by Emulate, are becoming more mainstream for predictive ADMET studies. ADMET Predictor by Simulations Plus, Inc., ADME/Tox by Sigma-Aldrich, LLC., PhysChem and ADME-Tox Prediction by ACD/Labs, PK/PD Database for Pharmacokinetic Properties by the Laboratory of Computational and Medicinal Chemistry, etc., are several tools developed for predicting toxicity related to ADME. Artificial intelligence (AI) may be used to simulate the ADME of drugs, determine if a therapeutic molecule will be effective in humans, and predict safety concerns.
During the period of post-COVID recovery, the current popularity and concurrent emergence of AI and machine learning (ML) are likely to have a substantial impact on drug design and development. These technologies have decreased the need for human intervention and raised the quality of life of people as they can be implemented in designing superior-quality healthcare goods. With the help of specialized molecular modeling techniques, such as in-silico simulation, pharmacophore modeling, molecular dynamics, virtual screening, and molecular docking, AI&ML-driven rational drug design seeks to explain the unforeseen bioactivity of natural products most perfectly in a limited timeframe.
Additionally, it describes the molecular interaction of drugs and their targets to create more effective drug leads. In January 2022, Charles River Laboratories and Valo Health, LLC, a technology firm leveraging human-centric data and AL-based computing, established a multi-year strategic collaboration to change the drug research & development process. A new revolutionary, AI-enabled drug discovery and development platform will be made available through this partnership by using Charles River's discovery optimization capabilities, including medicinal chemistry, ADME, biology, and pharmacology, and Valo's Opal Platform for small molecule development, which utilizes a`closed-loop`in a silico-experimental platform that rapidly iterates using program data to identify novel compounds. Thus, ADME testing based on automation, AI, and ML, among other technologies, is becoming an important part of the digital health boom, providing significant growth opportunities for the Pharma ADMET testing market.
Europe Pharma ADMET Testing Market Segmentation
The Europe pharma ADMET testing market is segmented by testing type, technology, application, and country. Based on testing type, the market is segmented into in vivo ADMET testing, in vitro ADMET testing, and in silico ADMET testing. The in vivo ADMET testing segment is dominating the market in 2022. Based on technology, the market is segmented into cell culture, high throughput, molecular imaging, and OMICS technology. The cell culture segment is dominating the market in 2022. Based on application, the market is segmented into systemic toxicity, renal toxicity, hepatotoxicity, neurotoxicity, and others. The systemic toxicity segment is dominating the market in 2022. Based on country, the market is segmented into Germany, the UK, France, Italy, Spain, and the Rest of Europe. Further, the Rest of Europe dominated the market in 2022.A few key players dominating the Europe pharma ADMET testing market are Agilent Technologies, Inc.; Bio-Rad Laboratories, Inc.; Biovia (Dassault Systèmes); Charles River Laboratories; CMIC HOLDINGS Co., LTD; Cyprotex Limited; IQVIA Inc.; MERCK KGaA; Promega Corporation; and Wuxi AppTec.
Table of Contents
Companies Mentioned
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Biovia (Dassault Systèmes)
- Charles River Laboratories
- CMIC HOLDINGS Co., LTD
- Cyprotex Limited
- IQVIA Inc.
- MERCK KGaA
- Promega Corporation
- Wuxi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 142 |
| Published | October 2022 |
| Forecast Period | 2022 - 2028 |
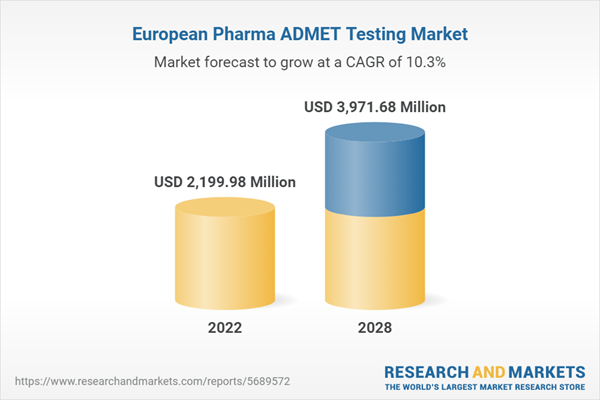
| Estimated Market Value ( USD | $ 2199.98 Million |
| Forecasted Market Value ( USD | $ 3971.68 Million |
| Compound Annual Growth Rate | 10.3% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 10 |