Speak directly to the analyst to clarify any post sales queries you may have.
Atopic dermatitis is the most common type of eczema, affecting approximately 26 million people in the United States. AD is children's most common skin disease, with prevalence steadily increasing from 8% to 12% in the last two decades. It is characterized by a defect in the skin barrier, which allows allergens and other irritants to enter the skin, leading to an immune reaction and inflammation. This reaction produces a red, itchy rash, most frequently occurring on the face, arms and legs, and the rash can cover significant areas of the body. The rash causes significant pruritus (itching), which can lead to damage caused by scratching or rubbing and perpetuating an ‘itch-scratch’ cycle. Pediatric patients with atopic dermatitis can suffer from sleep disturbances, behavioral problems, irritability, crying, and interference with normal childhood activities and social functioning. Adults with AD also frequently suffer from sleep disturbances, emotional impacts, and impaired social functioning. The author estimates that the prevalent cases of AD were high in China, followed by U.S. and Japan in 2020. The increasing prevalence of atopic dermatitis is driving the growth of the atopic dermatitis treatment market.
Atopic Dermatitis Diagnosis
- The exact cause of atopic dermatitis is unknown. Currently, no reliable biomarker which can distinguish the disease from other entities. However, the most commonly used biomarker is elevated total and/or allergen-specific serum IgE. The diagnosis of AD is based on the history and physical examination findings. Exposure to possible exacerbating factors, such as aeroallergens, irritating chemicals, foods, and emotional stress, should be investigated. Unfortunately, no specific laboratory findings or histologic features define atopic dermatitis.
Atopic Dermatitis Treatment
Most atopic dermatitis patients are treated with topical therapies, particularly low to mid-potency topical steroids and TCIs. These two classes of drugs constituted nearly all AD prescriptions. Despite their widespread use, existing topical therapies all possess substantial shortcomings:1. Topical corticosteroids, in combination with Vitamin D creams and antihistamines, are the first line of treatment for AD. The topical steroids aim to provide short-term itch relief to break the itch-scratch reflex cycle - the primary cause of reinfection. Although in more mild cases, these therapies may be effective, in moderate/severe cases, long-term use (of steroids) tends to do more harm than benefit. Prolonged use damages and dries out the epidermal layer of skin, which, somewhat paradoxically, ends up actually worsening the itch-scratch complex.
2. Topical steroids pose a particular concern in pediatric patients due to the risk of systemic absorption, the consequent risk of HPA axis suppression, and potential developmental problems. Chronic use of topical steroids in AD patients is generally avoided.
3. Second-line agents include immunosuppressant medications such as topical calcineurin inhibitors, including Pimecrolimus (Elidel) and Tacrolimus (Protopic). Topical calcineurin inhibitors are generally seen as providing less symptomatic improvement than topical steroids and are also associated with some application site burning. In 2005 the FDA placed a boxed warning on the labels of both TCIs regarding a potential increased risk of cancers, especially lymphomas.
4. Other treatments include biologics, typically starting with interleukin inhibitors such as tralokinumab-idrm, dupilumab, and nemolizumab.
5. Topical treatments include PDE-4 inhibitors such as Crisaborole (Eucrisa) and Difamilast (Moizerto); JAK inhibitors such as Ruxolitinib (Opzelura) and Delgocitinib (Corectim Ointment).
6. Oral JAK inhibitors such as Abrocitinib (Cibinqo), Upadacitinib (Rinvoq), and Baricitinib (Olumiant) are used for the treatment of atopic dermatitis.
ATOPIC DERMITIS: CLINICAL TRIALS SCENARIO
The clinical trial portfolio contains 153+ trials in various development phases. Most industry-sponsored drugs in active clinical development for AD are in the Phase II stage. The distribution of clinical trials across Phase I-IV indicates that the vast majority of trials for atopic dermatitis have been in the mid-phase of development, with 47% of trials in Phase I/II & II and only 29% in Phase Phase II/III-III.MARKET DRIVERS & TRENDS
Increasing Adoption for Biological Therapies
- Biological therapies such as anti-interleukin antibodies are widely used for treatment in the atopic dermatitis treatment market. With Dupixent being the biologic approved for atopic dermatitis in 2017, there is a demand for therapies with novel MOAs that are less immunogenic than current offerings. Many new classes of therapies are looking to enter the market over the next few years. The biologics such as interleukin inhibitors are making inroads into the atopic dermatitis market owing to better clinical profile and convenient patient dosing.
Anticipated Launch of Emerging Drugs
- A recent wave of biological products prescribed for treating AD will likely create a lucrative opportunity for growth in the atopic dermatitis treatment market. Despite the plethora of therapies currently available to patients with atopic dermatitis (AD), there is still room for improvement within the treatment space. The author anticipates the launch of five new agents among the 8MM, including two interleukin (IL) inhibitors (Lebrikizumab; CM-310), one topical Janus kinase (JAK) inhibitor (Delgocitinib), and two topical phosphodiesterase 4 (PDE-4) inhibitors (Roflumilast and JW-100). Launching these late-stage drugs will create an opportunity for growth in the global atopic dermatitis treatment market.
Focus on Novel Drugs with Novel Mechanism
- The therapeutic agents targeting the IL-13/IL-31 axis have been proven to be very effective in AD, some are already in the therapeutic armamentarium, and others are in development. Over the next five years, the publisher expects to see a surge of innovation emerging from the research and development pipeline and a range of technology-enabled transformations that will expand the evidence-basis for interventions and bring measurable improvements to outcomes.
SEGMENTATION ANALYSIS
- Drug Class: Topical Steroids are expected to account for a significant share of the global atopic dermatitis treatment market under the drug class segment. However, the other drug class is expected to be the fastest growing segment during the forecast period. Recently approved JAK and interleukin inhibitors are expected to drive the global atopic dermatitis therapeutics market during the forecast period.
Segmentation by Drug Class
- Topical Steroids
- Topical Calcineurin Inhibitors
- Other Class of Drugs
- Gender Type: Women are expected to dominate the global atopic dermatitis treatment market by gender type. This is because women have been at greater risk of atopic dermatitis in recent years.
Segmentation by Gender Type
- Men
- Women
- Age Group: People aged 19 years and above are expected to account for most of the global atopic dermatitis market share by the age group segment.
Segmentation by Age Group
- 18 years and Below
- 19 years and Above
- Severity Type: Among the severity type, People with mild form are expected to account for a significant share of the global market. However, people with moderate form are expected to be the fastest-growing segment during the forecast period.
Segmentation by Severity Type
- Moderate
- Mild
- Severe
GEOGRAPHICAL ANALYSIS
In North America, the United States dominates the global atopic dermatitis treatment market due to the rise in healthcare affordability in the country, a rise in the knowledge and awareness amongst the people, and a rise in technological advancement in this region. However, Japan is expected to grow faster with a high CAGR in the atopic dermatitis drug market due to the rise in access and quality of healthcare, increased awareness about disease management, and rising healthcare expenditure in this region.Segmentation by Geography
- North America
- U.S.
- APAC
- China
- Japan
- Europe
- France
- Germany
- UK
- Italy
- Spain
COMPETITIVE LANDSCAPE
- The global atopic dermatitis treatment market is dominated by many companies offering generic drugs and selective pharma/biotech companies offering patented/commercial drugs for treating atopic dermatitis. The U.S. Food and Drug Administration (FDA) approved the interleukin antibody Dupixent (Dupilumab) for treating AD in 2017. This marked the arrival of interleukin biologics in this treatment landscape. Recently the FDA approved interleukins such as Tralokinumab-idrm (adbry) in 2021, and PMDA approved Mitchga (Nemolizumab) in 2022 for treating atopic dermatitis. Although generic products continue to capture significant atopic dermatitis treatment market share, the arrival of biologics and other targeted therapies indicate a paradigm shift in this therapeutic space through the forecast period.
- The emerging therapeutics developed for atopic dermatitis include Lebrikizumab, CM-310, Tapinarof, Roflumilast, Rocatinlimab, and many others. Launching these novel emerging drugs will shift the atopic dermatitis therapeutics paradigm soon. With more than 153 molecules in various stages of development, it is expected that new vendors are likely to enter the market with a novel mechanism of action and better safety and efficacy profiles compared to the existing patented commercial drugs for treating atopic dermatitis
- Companies such as AbbVie, Pfizer, Novartis, LEO Pharma, Sanofi/Regeneron, Incyte Corp, Maruho Co., Ltd, Eli Lilly and Company, Japan Tobacco Inc, and others, etc., are the major players in the global atopic dermatitis treatment market. These players are focused on adopting growth strategies to strengthen their product portfolio.
- These companies have adopted strategies such as collaborations & acquisitions, expansion of geographical footprint, investments in research & development, and manufacture of novel drugs to compete in the global atopic dermatitis treatment market. Other prominent players operating in the global atopic dermatitis treatment market include Arcutis Biotherapeutics, Kyowa Hakko Kirin, Dermavant Sciences, Vanda Pharmaceuticals, CARA Therapeutics, and many others.
Key Vendors
- LEO Pharma
- Pfizer
- Sanofi/Regeneron
- Novartis
- Incyte Corp
- AbbVie
- Eli Lilly and Company
- Japan Tobacco Inc
- Maruho Co., Ltd
- Otsuka Holdings Co Ltd
- Astellas Pharma Inc
- Stiefel Laboratories
Other Prominent Vendors
- Arcutis Biotherapeutics
- Kyowa Hakko Kirin
- Vanda Pharmaceuticals
- Dermavant Sciences
- CARA Therapeutics
- AstraZeneca Plc (AstraZeneca)
- UNION therapeutics
- Asana
- Evelo Biosciences
- BiomX
- AOBiome Therapeutics, Inc.
- Amytrx
- RAPT Therapeutics
- Oneness Biotech
- VYNE’s Therapeutics
- BenevolentAI Ltd
- vTv Therapeutics Inc.
- Botanix Pharmaceuticals Ltd (BOT)
- selectION, Inc.
- Inmagene
- Novan, Inc.
REPORT COVERS
- Detailed overview of atopic dermatitis treatment market, including disease definition, classification, diagnosis, and treatment pattern
- Overview of the global trends of AD in the eight major markets (8MM)
- Historical, current, and projected patient pool of atopic dermatitis in the eight major markets (8MM) for 2018 - 2027
- In-depth market segment analysis, including products, treatment, and competitor analysis
- Atopic dermatitis treatment market share of the market players, company profiles, product specifications, and competitive landscape
- Comprehensive data on emerging trends, market drivers, growth opportunities, and restraints
- Detailed overview of marketed drugs with key coverage of developmental activities, including sponsor name, approved indication, territory, collaborations, licensing, mergers and acquisitions, regulatory designations, and other product-related activities
- Detailed overview of therapeutic pipeline activity and therapeutic assessment of the products by development stage, product type, route of administration, molecule type, and MOA type for Atopic Dermatitis across the complete product development cycle, including all clinical and non-clinical stages
- Detailed overview of clinical trial activity and therapeutic assessment of the products by development stage, product type, route of administration, molecule type, and geography type for atopic dermatitis across all clinical stages
- Coverage of dormant and discontinued pipeline projects along with the reasons across the atopic dermatitis treatment market
- Coverage of significant milestones (product approvals/launches timelines, clinical trial result publications, regulatory designations, licensing & collaborations, research & development progress of pipeline assets) in the atopic dermatitis space
KEY QUESTIONS ANSWERED
1. How big is the global atopic dermatitis treatment market?2. What is the growth rate of the global atopic dermatitis treatment market?
3. What factors drive the growth of the atopic dermatitis treatment market?
4. Who are the leading vendors operating in the global atopic dermatitis treatment market?
5. Which region holds the largest global atopic dermatitis treatment market share?
Table of Contents
Companies Mentioned
- LEO Pharma
- Pfizer
- Sanofi/Regeneron
- Novartis
- Incyte Corp
- AbbVie
- Eli Lilly and Company
- Japan Tobacco Inc
- Maruho Co., Ltd
- Otsuka Holdings Co Ltd
- Astellas Pharma Inc
- Stiefel Laboratories
- Arcutis Biotherapeutics
- Kyowa Hakko Kirin
- Vanda Pharmaceuticals
- Dermavant Sciences
- CARA Therapeutics
- AstraZeneca Plc (AstraZeneca)
- UNION therapeutics
- Asana
- Evelo Biosciences
- BiomX
- AOBiome Therapeutics, Inc.
- Amytrx
- RAPT Therapeutics
- Oneness Biotech
- VYNE’s Therapeutics
- BenevolentAI Ltd
- vTv Therapeutics Inc.
- Botanix Pharmaceuticals Ltd (BOT)
- selectION, Inc.
- Inmagene
- Novan, Inc.
Methodology
Our research comprises a mix of primary and secondary research. The secondary research sources that are typically referred to include, but are not limited to, company websites, annual reports, financial reports, company pipeline charts, broker reports, investor presentations and SEC filings, journals and conferences, internal proprietary databases, news articles, press releases, and webcasts specific to the companies operating in any given market.
Primary research involves email interactions with the industry participants across major geographies. The participants who typically take part in such a process include, but are not limited to, CEOs, VPs, business development managers, market intelligence managers, and national sales managers. We primarily rely on internal research work and internal databases that we have populated over the years. We cross-verify our secondary research findings with the primary respondents participating in the study.

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 230 |
| Published | November 2022 |
| Forecast Period | 2021 - 2027 |
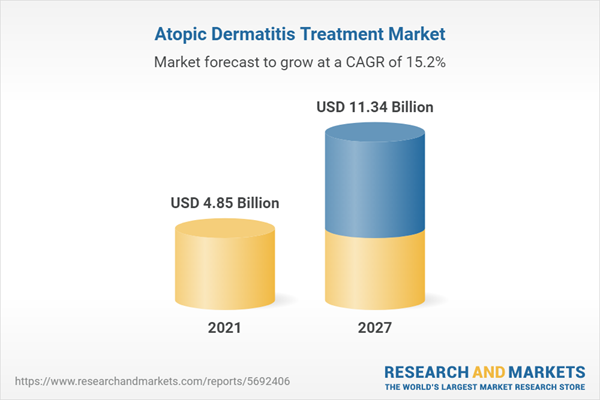
| Estimated Market Value ( USD | $ 4.85 Billion |
| Forecasted Market Value ( USD | $ 11.34 Billion |
| Compound Annual Growth Rate | 15.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 33 |