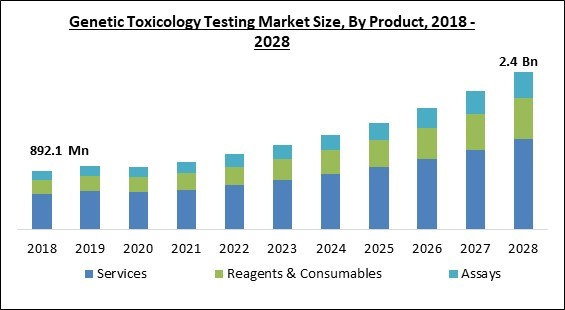
The Global Genetic Toxicology Testing Market size is expected to reach $2.4 billion by 2028, rising at a market growth of 13.2% CAGR during the forecast period.
The genetic toxicology testing research and observes the consequences of numerous physical, biological, and chemical substances on the heredity of live creatures. Genetic toxicology is the study of the harmful outcomes of DNA damage. These examinations are carried out either to detect chromosomal damage or gene mutation.
Agrochemicals, pharmaceutical impurities, chemicals utilized in the cosmetics industry, pharmaceutical medications, and industrial chemicals are all subjected to toxicological studies. Genetic toxicology is a crucial component of research and development (R&D), where novel medications are developed and designed through a variety of studies. These tests aid in the development of novel medications and vaccinations.
These studies also concentrate on in vitro drug discovery and tailored pharmaceuticals. In addition to these applications, there is a growing need for humanized animal models, which will present a plethora of chances for the major competitors in the genetic toxicology testing market. Testing for genetic toxicity has a variety of uses in the cosmetics, food, pharmaceutical, and biotech industries.
The requirement for genetic toxicology information is predicted to increase due to the increased medication research and discovery as well as the expanding usage of pharmacogenomics. This is because it can evaluate the substances that can cause chromosomal damage or genetic alterations. A significant growth factor in the genetic toxicity testing field is also the development of facilities and the availability of funding globally.
The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include Thermo Fisher Scientific, Inc., Charles River Laboratories International, Inc., Laboratory Corporation of America Holdings, Eurofins Scientific Group, Jubilant Pharmova Limited., Syngene International Limited, Gentronix Ltd., Inotiv Inc., Creative Bioarray and MB Research Laboratories.
The genetic toxicology testing research and observes the consequences of numerous physical, biological, and chemical substances on the heredity of live creatures. Genetic toxicology is the study of the harmful outcomes of DNA damage. These examinations are carried out either to detect chromosomal damage or gene mutation.
Agrochemicals, pharmaceutical impurities, chemicals utilized in the cosmetics industry, pharmaceutical medications, and industrial chemicals are all subjected to toxicological studies. Genetic toxicology is a crucial component of research and development (R&D), where novel medications are developed and designed through a variety of studies. These tests aid in the development of novel medications and vaccinations.
These studies also concentrate on in vitro drug discovery and tailored pharmaceuticals. In addition to these applications, there is a growing need for humanized animal models, which will present a plethora of chances for the major competitors in the genetic toxicology testing market. Testing for genetic toxicity has a variety of uses in the cosmetics, food, pharmaceutical, and biotech industries.
The requirement for genetic toxicology information is predicted to increase due to the increased medication research and discovery as well as the expanding usage of pharmacogenomics. This is because it can evaluate the substances that can cause chromosomal damage or genetic alterations. A significant growth factor in the genetic toxicity testing field is also the development of facilities and the availability of funding globally.
COVID-19 Impact Analysis
Due to lockdowns that have halted manufacturing operations and enforced travel restrictions, affecting the transit of shipments, the rapid expansion of COVID-19 has resulted in a lack of medical equipment and supplies. COVID-19 has not severely affected the availability of raw materials used in genetic toxicology, with the exception of transportation-related delays brought on by travel restrictions and labor scarcity difficulties. Toxicology research was being conducted to comprehend the virus mutation. Similar to this, many researchers used genotoxic to evaluate how well medications worked on the COVID-19 virus. Therefore, it can be said that the pandemic had a positive impact on the genetic toxicology testing market.Market Growth Factors
Rising investments in pharmaceutical R&D by companies
By raising the adoption of substances in the preclinical phases, R&D operations primarily aim to raise the overall likelihood of acceptance of Phase I therapeutic candidates. In the initial stages of medication development, intense R&D activities are carried out to accomplish this. The necessity for genetic toxicology research is consequently increased. Before a medicine enters the pricey clinical stages, higher R&D expenditures in the early phases of drug products are also anticipated to enhance the usage of in vivo toxicity techniques.Increasing demand for humanized methods of in vivo testing including animals
More and more biomedical research applications use humanized mice as test subjects. Professors at Yale University and the Jackson Laboratory (US) were awarded a three-year grant in 2017 to perform advanced research on humanized MISTRG models for understanding the biology of human melanoma along with identifying therapeutic targets. The Connecticut Bioscience Innovation Fund (CBIF) awarded the Jackson Laboratory and Yale University (US) a grant in the amount of USD 700,000 in November 2018. This award supported scientific collaboration to create humanized mouse models that correctly reflect how people react to disease and cancer treatments.Marketing Restraining Factor:
Insufficient verified in vitro models for complex endpoint research
Given that human proteins and antibodies make over half of the novel medications created, autoimmune disease and immunological activation are crucial endpoints for drug development. These medicinal compounds can only yet be tested in animal models. The use of in vitro genetic toxicology testing techniques does not permit the assessment of the response induced within a body following secondary infection. These techniques can't assess how well a body recovers in response to short-term versus long-term immunosuppression.Product Outlook
Based on product, the genetic toxicology testing market is classified into reagents & consumables, assays, and services. The service segment procured the largest revenue share in the genetic toxicology testing market in 2021. The segment expansion is being fuelled by Contract Research Organizations' (CROs') growing strategic ambitions through investment in this sector. The rising scope of services in the epidermis and genetic toxicological testing is another factor fuelling the service sector. For instance, in order to meet the growing demand around the world, Gentronix announced that the scope of its services in the field of cutaneous toxicity will be expanding.Type Outlook
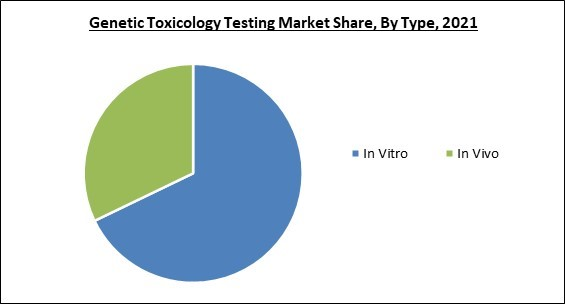
On the basis of type, the genetic toxicology testing market is bifurcated into In Vitro and In Vivo. The in vivo segment procured a significant revenue share in the genetic toxicology testing market in 2021. An essential component of medical research generally is in vivo testing, particularly in clinical trials. Studies conducted in living organisms (in vivo) offer useful knowledge about a substance's effects or the progression of a disease. Clinical trials and animal studies are the two main categories of in vivo testing.Application Outlook
By application, the genetic toxicology testing market is segmented into pharmaceutical & biotechnology, food industry, cosmetics industry, and Others. The pharmaceutical and biotechnology segment witnessed the maximum revenue share in the genetic toxicology testing market in 2021. It is projected that one of the driving forces behind the rise will be the extensive utilization of genotoxicity testing in medicine development and research. Testing for genetic toxicity is done at the preclinical stage. Additionally, it is predicted that the biotechnology and pharmaceutical sector will increase at a fast pace as well. Given that testing is necessary for all quantities of medications and substances.Regional Outlook
Region-wise, the genetic toxicology testing market is analysed across North America, Europe, Asia Pacific and LAMEA. The North American region witnessed the highest revenue share in the genetic toxicology testing market in 2021. The dominance in the region can be due to rising medication development and significant pipeline spending. Additionally, the presence of important actors in the region makes genotoxicology testing more accessible for local pharmaceutical and biotech firms. The significant share of this industry is due to the growth of structure-based medication designs, rising financing for life sciences research, high R&D costs in the biopharmaceutical industry, and the burgeoning uptake of cutting-edge techniques in North America.The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include Thermo Fisher Scientific, Inc., Charles River Laboratories International, Inc., Laboratory Corporation of America Holdings, Eurofins Scientific Group, Jubilant Pharmova Limited., Syngene International Limited, Gentronix Ltd., Inotiv Inc., Creative Bioarray and MB Research Laboratories.
Strategies Deployed in Genetic Toxicology Testing Market
- Feb-2022: Eurofins Clinical Testing Lux Sarl, a subsidiary of Eurofins Scientific acquired Genetic Testing Service, (Gentis), the market leader in specialized genetic analysis in Vietnam. With the acquisition, Eurofins focused on expanding its reach in Asia and complemented its worldwide network of clinical diagnostics laboratories working on technical and advanced genetic testing.
- Jan-2022: Inotiv acquired Integrated Laboratory Systems, a portfolio company of Sier Capital Partners. Through the acquisition, Inotiv aimed to diversify the company's in vivo and in vitro toxicology services, comprising of prominent pathology and toxicology expertise. The acquisition increased the company's services into genomics, bioinformatics, and computational toxicology which further extended its market reach and created effective new cross-selling possibilities.
- Sep-2021: Labcorp took over operating assets and intellectual property (IP) of the autoimmune business unit of Myriad Genetics, together with Vectra rheumatoid arthritis (RA) assay. The inclusion of Vectra testing abilities to Labcorp products' range presented the tremendous potential for the company to develop the test’s availability and make Labcorp a single-source diagnostics solution for RA providers.
- Aug-2021: Inotiv announced the acquisition of Gateway Pharmacology Laboratories, a preclinical contract research organization specializing in cardiovascular pharmacology studies. Following this acquisition, Inotiv augmented its array of in vivo abilities and integrated laboratory support assistance to include cardiovascular and renal pharmacology.
- Jul-2021: Gentronix took over Big Blue transgenic mutation assay models by Bioreliance, the largest provider of outsourcing services focused on the rapidly growing biologics sector of the pharmaceutical industry. Through this acquisition, Gentronix aimed to double its revenues and augment its genetic toxicology capabilities in vitro skin and in vitro ocular toxicity testing.
- Jun-2021: Charles River Laboratories acquired Vigene Biosciences, a premier, a gene therapy contract development and manufacturing organization. Through the acquisition, Charles River focused on augmenting its extensive cell and gene therapy range by utilizing the gene therapy expertise of Vigene Biosciences. The increased portfolio span incorporated each of the major CDMO platforms - cell therapy, viral vector, and plasmid DNA production.
- Jan-2021: Charles River Laboratories partnered with Cypre, a biotechnology company that utilizes 3D hydrogel technology to advance the knowledge of the tumor microenvironment and predict therapeutic usefulness. This partnership helped the former company's clients with access to Falcon-X, Cypre’s proprietary 3D tumor model platform. Moreover, the partnership broadened Charles River’s 3D in vitro testing services to further optimize immuno-oncological methods for its clients.
- Jan-2019: Charles River Laboratories formed an agreement with Toxys, a company that provides innovative in vitro toxicity screening solutions. Under the agreement, CRL focused on providing ToxTracker, a range of assays that enable rapid carcinogenicity toxicity hazard identification in novel and existing drugs, chemicals, and other substances in North America. The agreement equipped CRL's client base with the utilization of the ToxTracker assay that can assist in mitigating the risk of regulatory rejection by illustrating the exact mechanism of genotoxicity.
Scope of the Study
By Product
- Services
- Reagents & Consumables
- Assays
By Type
- In Vitro
- In Vivo
By Application
- Pharmaceutical & Biotechnology
- Food Industry
- Cosmetics Industry
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Thermo Fisher Scientific, Inc.
- Charles River Laboratories International, Inc.
- Laboratory Corporation of America Holdings
- Eurofins Scientific Group
- Jubilant Pharmova Limited.
- Syngene International Limited
- Gentronix Ltd.
- Inotiv Inc.
- Creative Bioarray
- MB Research Laboratories
Unique Offerings
- Exhaustive coverage
- The highest number of market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology
Chapter 2. Market Overview
Chapter 4. Global Genetic Toxicology Testing Market by Product
Chapter 5. Global Genetic Toxicology Testing Market by Type
Chapter 6. Global Genetic Toxicology Testing Market by Application
Chapter 7. Global Genetic Toxicology Testing Market by Region
Chapter 8. Company Profiles
Companies Mentioned
- Thermo Fisher Scientific, Inc.
- Charles River Laboratories International, Inc.
- Laboratory Corporation of America Holdings
- Eurofins Scientific Group
- Jubilant Pharmova Limited.
- Syngene International Limited
- Gentronix Ltd.
- Inotiv Inc.
- Creative Bioarray
- MB Research Laboratories
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | October 2022 |
| Forecast Period | 2021 - 2028 |
| Estimated Market Value ( USD | $ 1018 Million |
| Forecasted Market Value ( USD | $ 2388 Million |
| Compound Annual Growth Rate | 13.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |