Bioprinting Industry Revenues to Reach Almost $1.2 Billion by 2028
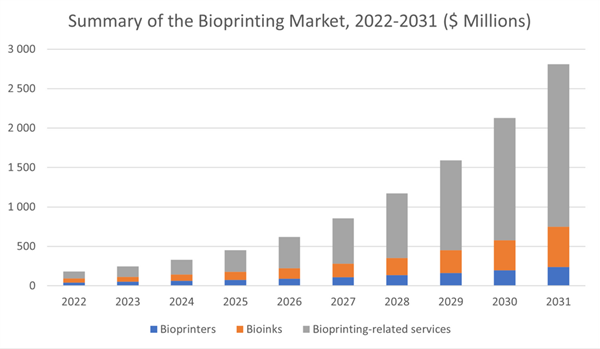
Revenues from bioprinting are expected to reach almost $1.2 billion in 2028, compared with just $182 million today. By 2028, the analyst expects almost 70 percent of the bioprinting industry to come from applications and service revenues. The new report, “Bioprinting: Markets and Opportunities”, claims that we are many years from transplanting printed organs. Nonetheless, bioprinting is finding a rapidly growing application in drug and cosmetics testing. Using printed tissues and organs avoids the need for animal testing and enables testing on printed tissue and organs that are realistic substitutes for the real thing.
Report Scope:
The analyst has been tracking the bioprinting industry for almost a decade and this is its third major bioprinting report. The report covers bioinks and bio-printing related applications, as well as the bioprinters themselves. Applications discussed in the report include drug discovery and testing, cosmetics testing, tissue and bone printing. We also examine the long-term prospects for transplanting bioprinted organs. And there is a chapter on new directions for bioprinting including discussions on soft robotics, pill printing, and printed food.
The new report provides a realistic assessment of bioprinting’s commercial future including an analysis of how the major bioprinting companies are designing their marketing strategies. In addition to the pure play bioprinting companies, the bioprinting sector is seeing involvement from major life science companies and 3D printing companies. There are now well over 100 firms in the bioprinting sector, but most of these companies have negligible revenues. Among the firms that are discussed in this report are 3DBio Therapeutics, 3D Systems/Systemic Bio, BICO (Cellink), Collplant, Cyfuse, EnvisionTEC/Desktop Metal, FluidForm, Inventia Life Science, Organovo Holdings, Poietis, Regamat, Rokit Healthcare and many others.
According to Lawrence Gasman, author of the report, “New applications are quickly emerging for bioprinting, and they are very much in line with important megatrends in healthcare - notably the trends towards personalized medicine and bringing care closer to the patient. For example, bioprinting can enable procedures such as skin grafting to be performed in a clinic rather than at specialized hospitals.”
Report Highlights
- Production-grade bioprinters and reproducible bioprinting applications are coming to the fore. By 2031, R&D service revenues will clock up almost $250 million in service/applications revenue for bioprinting, but this will still only amount to 12 percent of total service/applications revenues in the bioprinting industry.
- The analyst expects technological innovation across the entire bioprinting sector to accelerate. The new report points to three developments in particular as areas of strong commercial potential. One of these is the arrival of organs-on-a-chip, a product class that bioprinting does much to enable. The report also predicts that bioprinters will move beyond the currently dominant extrusion and inkjet machines. Already as many as 20 novel ways of doing bioprinting are emerging from leading labs and healthcare facilities worldwide. While extrusion and inkjet bioprinters will account for well over 90 percent of bioprinter revenues in 2022, by 2031, 15 percent of bioprinter revenues will come from printers based on entirely novel printing technologies.
- Another technical trend that is leading bioprinting forward technologically and will improve costs to patients and expand capabilities is the trend toward next-generation bioprinting platforms that have greater capacities, automate much of the work, and integrate medical functionality well beyond basic bioprinting. By 2031 the analyst expects production grade bioprinters or biomanufacturing platforms incorporating bioprinting to clock up around $100 million in revenues.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3DBio Therapeutics
- 3D Systems/Systemic Bio
- 3DBio Therapeutics
- BICO (Cellink)
- Collplant
- Cyfuse
- EnvisionTEC/Desktop Metal
- FluidForm
- Inventia Life Science
- Organovo Holdings
- Poietis
- Regamat
- Rokit Healthcare