Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Rising Incidence of Cardiovascular and Peripheral Arterial Diseases
The global burden of cardiovascular disease (CVD) continues to be a leading cause of death and disability. According to the World Health Organization (WHO), CVDs account for over 17.9 million deaths annually, making them the top cause of mortality worldwide. A substantial proportion of these cases involve atherosclerosis, coronary artery disease (CAD), and peripheral artery disease (PAD)all conditions for which balloon catheters are a critical component of diagnosis and treatment, particularly in angioplasty procedures.Balloon catheters help open narrowed or blocked blood vessels without requiring more invasive surgeries, which has made them highly favored in both emergency and elective interventions. In regions with aging populations, especially North America and parts of Europe, the demand for minimally invasive vascular procedures has steadily increased due to the higher prevalence of hypertension, diabetes, and obesity.
Key Market Challenges
High Cost of Advanced Devices and Procedures
One of the most pressing challenges facing the global advanced balloon catheter market is the high cost associated with these devices, which can limit their accessibility, especially in low- and middle-income countries. Drug-coated and specialty catheters are significantly more expensive than conventional balloon catheters, making their widespread use a financial burden for both healthcare systems and patients. Even in high-income countries, insurance coverage does not always fully reimburse advanced interventional procedures. In publicly funded healthcare systems, budget constraints and cost-containment policies often result in delayed or limited adoption of newer, costlier technologies. The challenge is further compounded by the need for specialized training and infrastructure, which increases the total cost of ownership for hospitals and surgical centers.Government funding programs, while helpful, are often insufficient to address the gap in demand for cutting-edge equipment versus what is affordable or reimbursable. This disparity limits the penetration of advanced balloon catheter technology in various parts of the world, hindering equitable healthcare delivery.
Key Market Trends
Growing Use of Drug-Coated Balloons (DCBs)
Drug-coated balloons are gaining significant traction as they offer a stent-less solution for revascularization. These devices are particularly useful in treating in-stent restenosis (ISR) and femoral-popliteal lesions, where stents may not be ideal due to anatomical or movement-related factors. The appeal of DCBs lies in their ability to deliver therapeutic agents like paclitaxel directly into the vessel wall during a short inflation time, reducing the risk of chronic inflammation and improving healing outcomes.Healthcare systems are showing increasing interest in DCBs, especially for peripheral interventions. Clinical trials supported by government agencies such as the National Institutes of Health (NIH) in the U.S. have investigated the long-term efficacy and safety of these devices, providing a research-backed push toward greater adoption. This trend is expected to grow further as more biocompatible and biodegradable drug coatings are introduced and regulatory bodies clarify safety profiles, making DCBs a mainstay in future vascular treatments.
Key Market Players
- Abbott Laboratories
- Boston Scientific Corporation
- Medtronic plc
- Terumo Corporation
- MicroPort Scientific Corporation
- B. Braun Melsungen AG
- Meril Life Sciences Pvt. Ltd.
- Hexacath
- Tokai Medical Products Inc.
- Cook Medical Inc.
Report Scope:
In this report, the Global Advanced Balloon Catheter Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Advanced Balloon Catheter Market, By Raw Material Type:
- Nylon
- PET
- Polyurethane
- Others
Advanced Balloon Catheter Market, By Application:
- Coronary Procedures
- Peripheral Procedures
- Neurovascular Procedures
- Other Procedures
Advanced Balloon Catheter Market, By End User:
- Hospitals and Ambulatory Surgical Centres
- Catheterization Laboratories
- Others
Advanced Balloon Catheter Market, By Region:
- North America
- United States
- Mexico
- Canada
- Europe
- France
- Germany
- United Kingdom
- Italy
- Spain
- Asia-Pacific
- China
- India
- South Korea
- Japan
- Australia
- South America
- Brazil
- Argentina
- Colombia
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Advanced Balloon Catheter Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Boston Scientific Corporation
- Medtronic plc
- Terumo Corporation
- MicroPort Scientific Corporation
- B. Braun Melsungen AG
- Meril Life Sciences Pvt. Ltd.
- Hexacath
- Tokai Medical Products Inc.
- Cook Medical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | August 2025 |
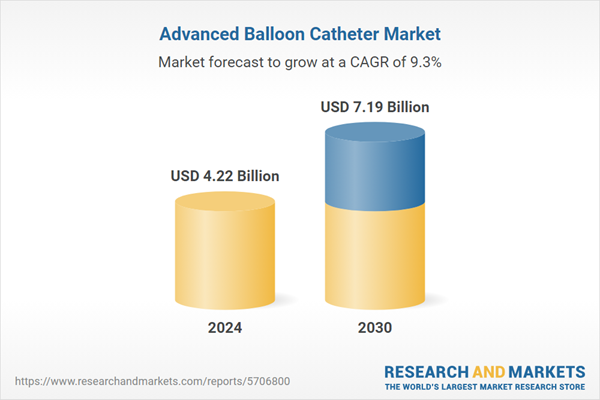
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 4.22 Billion |
| Forecasted Market Value ( USD | $ 7.19 Billion |
| Compound Annual Growth Rate | 9.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |