Eczema, commonly referred to as atopic dermatitis, is a skin ailment that affects the skin and results in itching, dryness, and inflammation. The illness has a potential for chronicity and occasionally flares up and spreads. Drugs for atopic dermatitis, such as atopic eczema steroids, are utilized to treat eczema, lessen itching, and treat other atopic dermatitis-related disorders.
Atopic dermatitis patients run the risk of acquiring asthma, hay fever, and food allergies. Regular moisturizing and other skin care practices help reduce itching and stop future outbreaks. Medicated lotions or ointments may also be used during treatment. It causes skin that is scratchy, red, swollen, and cracked. The damaged sites may leak a clear fluid that thickens over time.
Although the disorder can strike at any age, it usually first manifests in childhood and varies in severity throughout time. Much of the body may be impacted in youngsters under the age of one year. The areas on the inside of the knees and elbows are most frequently affected as kids age. Adults are more frequently afflicted in the hands and feet.
Scratching the affected regions makes the symptoms worse, and individuals who are scratching have a higher chance of developing skin infections. Atopic dermatitis frequently leads to the development of asthma or hay fever. Avoiding things that aggravate the illness, regular bathing followed by the use of moisturizing cream, using steroid creams when flare-ups happen, and drugs to relieve itching are all part of the treatment.
Wool garments, soaps, fragrances, dust, chlorine, and cigarette smoke are a few things that frequently aggravate it. Some people may benefit from phototherapy. If other treatments are ineffective, steroid tablets or lotions based on calcineurin inhibitors may occasionally be employed. If a bacterial infection manifests, antibiotics (by mouth or topically) could be required. Only if food allergies are detected are dietary modifications required.
COVID-19 Impact Analysis
The coronavirus is still creating trouble in people's lives on a worldwide scale, disrupting everything from social interactions to how they seek medical care and see the outside world. Every industry has experienced a profit or output decrease as a result of the pandemic epidemic. However, the market for treating atopic dermatitis has anticipated a substantial impact because of the irregularity of hospital visits by patients. Teledermatology has grown during the crisis when the majority of patients continue to receive generalized care and/or additional consultative therapies without seeing a doctor in person.Market Growth Factors
Growing Demand For Biologics
The treatment of dermatological disorders, particularly atopic dermatitis, has changed as a result of the use of biologic drugs in dermatology. Biologics have demonstrated a good response to atopic dermatitis, improving the overall quality of life. As a result, there is now a greater demand for biologic treatments for atopic dermatitis. In the upcoming several years, this pattern is probably going to continue. For the time being, only one biologic product has been authorized for the treatment of atopic dermatitis. Many more are currently through phase II clinical studies and will probably be approved soon.Rising Prevalence Of Atopic Dermatitis
The increasing prevalence of atopic dermatitis medications around the world is anticipated to be the main factor driving the market for atopic dermatitis medications. A large number of people suffer with atopic dermatitis which is expected to rise even more in upcoming years. Geographical location, climatic circumstances, socioeconomic level, lifestyle, age, gender, inheritance, and individual behaviors all have an impact on the prevalence of dermatological problems around the world. An individual with atopic dermatitis must deal with allergies on a daily basis.Market Restraining Factors
Side Effects Of Topical Steroids
Topical steroid treatments lasting fewer than four weeks are often risk-free and trouble-free. Long-term topical steroid use or frequent repetition of short doses of harsher steroids may cause issues. The primary issue is the prolonged usage of potent steroids. Mild topical steroids rarely have side effects. Topical steroids may have local or systemic side effects. Systemic refers to an issue that affects the entire individual, whereas local refers to that particular area of skin.Class Outlook
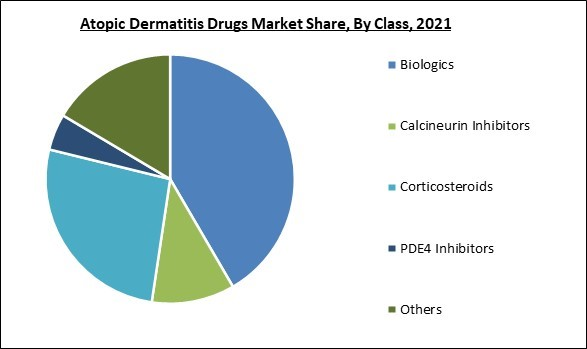
Based on class, the atopic dermatitis drugs market is segmented into corticosteroids, calcineurin inhibitors, PDE4 inhibitors, biologics and others. In 2021, the corticosteroids segment acquired a substantial revenue share in the atopic dermatitis drugs market. Many conditions, such as inflammatory bowel disease, rashes,and asthma, can be successfully treated with corticosteroid medications, such as cortisone, hydrocortisone, and prednisone.Route Of Administration Outlook
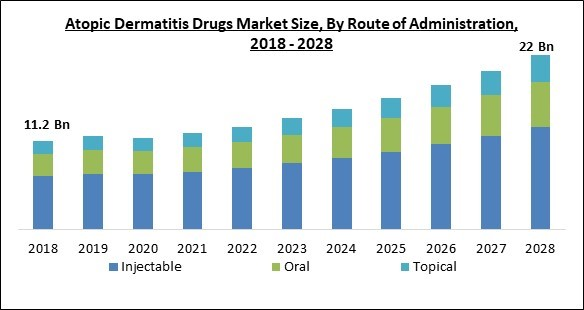
On the basis of route of administration, the atopic dermatitis drugs market is fragmented into topical, injectable and oral. In 2021, the injectable segment held the largest revenue share in the atopic dermatitis drugs market. This is due to the rising popularity of injectable drugs like Dupilumab. Tofacitinib and ruxolitinib are other injectable biologics being studied in clinical trials. In the near future, biologics are anticipated to alter the therapy landscape for AD.Regional Outlook
Region wise, the atopic dermatitis drugs market is analyzed across North America, Europe, Asia Pacific and LAMEA. In 2021, the North America region led the atopic dermatitis drugs market by generating the highest revenue share. The availability of a well-established R&D infrastructure and kind reimbursement rules are two major factors contributing to the region's sustained dominance. Additionally, the regulatory and reimbursement environment is constantly changing to keep up with the rapid advancement of research in this field.The Cardinal Matrix - Atopic Dermatitis Drugs Market Competition Analysis
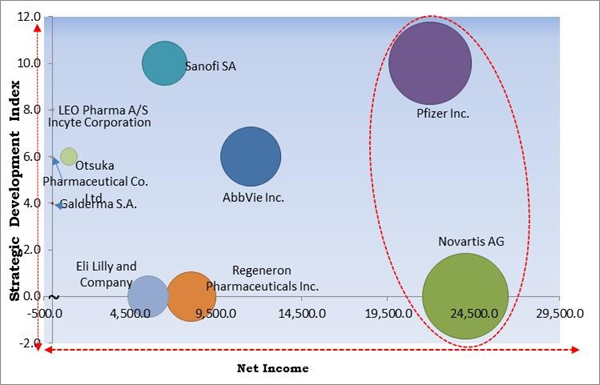
The major strategies followed by the market participants are Acquisitions. Based on the Analysis presented in the Cardinal matrix; Pfizer, Inc. and Novartis AG are the forerunners in the Atopic Dermatitis Drugs Market. Companies such as AbbVie, Inc., Sanofi SA, Regeneron Pharmaceuticals Inc. are some of the key innovators in Atopic Dermatitis Drugs Market.
The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include Pfizer Inc., Sanofi SA, AbbVie Inc., Galderma S.A., Eli Lilly and Company, Leo Pharma A/S, Regeneron Pharmaceuticals Inc., Otsuka Pharmaceutical Co. Ltd., Novartis AG, and Incyte Corporation.
Strategies Deployed in Atopic Dermatitis Drugs Market
Mergers and Acquisitions:
- Oct-2022: Incyte completed the acquisition of Villaris Therapeutics, an asset-centric biopharmaceutical company. This acquisition would complement Incyte's current portfolio, providing Incyte the opportunity to improve treatment options for people with vitiligo, that leverage the expertise established in the dermatology space.
- Jun-2022: Pfizer took over ReViral, a privately held, clinical-stage Biopharmaceutical Company. This acquisition further demonstrates Pfizer's commitment to advancing pioneering science both through its in-house expertise and work with leading, innovative companies - with the aim to deliver breakthroughs to patients suffering from severe infectious diseases.
- Mar-2022: Pfizer completed the acquisition of Arena Pharmaceuticals, an American biopharmaceutical company. Through this acquisition, Pfizer would add the impressive experience and pipeline of Arena Pharmaceuticals to Pfizer’s Inflammation & Immunology therapeutic area, helping the company further its purpose of developing breakthroughs to change the lives of those with immuno-inflammatory diseases.
- Jan-2022: Galderma took over ALASTIN Skincare, a specialty aesthetics company. This acquisition aimed at enhancing Galderma’s integrated dermatology platform with a comprehensive collection of scientifically-approved products for daily skincare regimens and peri-procedural use. Also, the acquisition would underscore Galderma’s commitment to being the partner of choice for aesthetic professionals.
- Dec-2021: AbbVie took over Soliton, a medical device company. The acquisition would complement Allergan Aesthetics' portfolio of non-invasive body contouring treatments to include a proven treatment for the appearance of cellulite.
- Nov-2021: Sanofi completed the acquisition of Translate Bio, a biotechnology company that specializes in biotechnology, RNA therapeutics, and rare diseases. The acquisition support Sanofi’s mRNA Center of Excellence, which plans to explore the potential of enhanced mRNA vaccines & other key areas that includes immunology, oncology, and rare diseases.
- Nov-2021: Sanofi took over Kadmon Holdings, which operates as a biopharmaceutical company. This acquisition would strengthen the expansion & growth of the general medicines portfolio.
- Jul-2019: LEO Pharma completed the acquisition of Bayer. LEO Pharma advances significantly towards its goal of helping 125 million patients by 2025. With this acquisition, the LEO considerably improve its size in key markets like Austria, Brazil, and South Africa - underlining its ambition to become a global leader in medical dermatology.
Approvals and Trials:
- Oct-2022: LEO Pharma got European approval for Adtralza, a drug to treat moderate-to-severe AD in adolescents. The approval would address LEO's relentless commitment to bringing the latest treatment options for those living with atopic dermatitis. This approval enables LEO to address the unmet needs of a broader patient population as it looks forwards to work with key stakeholders across the Europe region.
- Jun-2022: Sanofi got FDA approval for Dupixent, a biologic medicine approved to treat moderate-to-severe atopic dermatitis from infancy to adulthood. The approval would enable the availability of Dupixent for young people with the disease.
- Jan-2022: Pfizer got FDA approval for CIBINQO, an oral, once-daily, Janus kinase 1 (JAK1) inhibitor. CIBINQO would offer the treatment of adults living with refractory, moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with other systemic drug products. CIBINQO would provide an important latest oral option that would help those who have yet to find relief.
- Jan-2022: AbbVie got FDA approval for RINVOQ, a once-daily oral option that can significantly enhance the debilitating itch & skin symptoms of atopic dermatitis. Through this acquisition, AbbVie would continue its efforts to enhance care in this disease state & other chronic, immune-mediated conditions.
- Dec-2021: Leo Pharma got FDA approval for Adbry, an eczema drug. The injected antibody drug would address adults and older who don’t get proper relief from topical prescription therapies. Adbry would signify important progress in LEO's aim of enhancing the standard of care in medical dermatology.
- Sep-2021: Otsuka got FDA approval for Moizerto Ointment, a treatment for atopic dermatitis. Otsuka expects that Moizerto Ointment would serve as the latest treatment option for people having atopic dermatitis in Japan.
- Sep-2021: Incyte got FDA approval for Opzelura, a topical JAK inhibitor to treat AD. With this approval, the company looks forward to bringing Opzelura to the patient community and also continuing to explore its potential in other skin diseases.
Scope of the Study
By Route of Administration
- Injectable
- Oral
- Topical
By Class
- Biologics
- Calcineurin Inhibitors
- Corticosteroids
- PDE4 Inhibitors
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Pfizer Inc.
- Sanofi SA
- AbbVie Inc.
- Galderma S.A.
- Eli Lilly and Company
- Leo Pharma A/S
- Regeneron Pharmaceuticals Inc.
- Otsuka Pharmaceutical Co. Ltd.
- Novartis AG
- Incyte Corporation
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Sanofi SA
- AbbVie Inc.
- Galderma S.A.
- Eli Lilly and Company
- Leo Pharma A/S
- Regeneron Pharmaceuticals Inc.
- Otsuka Pharmaceutical Co. Ltd.
- Novartis AG
- Incyte Corporation