Speak directly to the analyst to clarify any post sales queries you may have.
How integrative omics modalities and rigorous operational practices are reshaping trial design pipelines and translational strategies across therapeutic development
The convergence of high-throughput molecular profiling, advanced bioinformatics, and pragmatic clinical operations has elevated omics-based clinical trials from niche initiatives to foundational elements of precision medicine development. Omics modalities, including genomics, transcriptomics, proteomics, and metabolomics, are increasingly embedded into trial protocols to enable patient stratification, biomarker discovery, response monitoring, and adaptive decision-making. This integration is reshaping endpoint definition and powering hypothesis-driven trial designs that align therapeutic mechanisms with molecular subtypes.
Across stakeholders, there is a growing recognition that omics data must be collected, curated, and analyzed with operational rigor equivalent to primary clinical endpoints. Consequently, trial teams are adopting standardized sample handling protocols, validated assay workflows, and data governance frameworks that support reproducibility and regulatory acceptability. These practices are complemented by advances in decentralized sampling, digital phenotyping, and cloud-native analytics, which together expand participant access and accelerate data availability for interim decision gates.
Transitioning safely from exploratory biomarkers to clinically validated companion diagnostics requires coordinated investment in analytical validation, clinical validation, and cross-functional regulatory engagement. Sponsors who align translational science with pragmatic operational planning position their programs to reduce late-stage attrition and to extract higher value from each enrolled cohort. The introduction sets the stage for the deeper thematic analysis that follows, focusing on transformative shifts, tariff-driven headwinds, segmentation nuances, regional variability, and actionable recommendations for industry leaders.
Key technological, operational, and collaborative inflection points that are rapidly accelerating the adoption and clinical utility of omics in trial development
Several transformative shifts are altering the landscape of omics-enabled clinical trials, driven by technological, regulatory, and operational inflection points. First, assay throughput and cost-per-sample improvements have lowered barriers to routine multi-omic profiling, enabling broader incorporation of molecular endpoints into Phase I and Phase II studies for hypothesis generation and adaptive enrichment.
Second, analytic maturity has advanced from bespoke bioinformatics scripts to validated, reproducible pipelines that support regulatory dialogue and cross-site comparability. This standardization reduces analytical variability and permits earlier reliance on molecular signals for adaptive randomization and biomarker-driven cohort expansion. Third, decentralized and hybrid trial models are reducing barriers to patient participation and improving sample diversity, which enhances the generalizability of molecular findings and mitigates traditional enrollment bottlenecks.
Fourth, the rise of cross-sector collaborations among academic centers, specialized diagnostic laboratories, and contract research providers is enabling integrated service models where sample processing, centralized analytics, and regulatory-ready documentation are delivered as coordinated workflows. Finally, payer and HTA conversations increasingly factor molecular stratification into value assessments, pressuring sponsors to generate robust clinical and health economic evidence for biomarker-driven indications. Collectively, these shifts are moving omics from an exploratory research tool to an essential operational component that can materially affect development timelines and clinical decision-making frameworks.
Assessing how changes in US tariff policy can reshape procurement dynamics, supply chain resilience, and operational continuity for omics-enabled clinical trials
Anticipated tariff policy shifts and trade measures originating from the United States can impose tangible upstream and downstream impacts on omics-enabled trial operations in several domains. Tariffs on laboratory equipment, reagents, consumables, and clinical devices can elevate procurement costs and complicate supplier selection, prompting sponsors and service providers to reassess existing vendor contracts and to diversify sourcing strategies. Where single-source reagents or platforms are affected, laboratories may face temporary validation gaps while transitioning to alternative supplies, creating potential interruptions in assay continuity and data comparability.
Further, tariffs that affect imported diagnostic kits or specialized sequencing flow cells can extend lead times and increase inventory carrying costs for diagnostic labs and contract research organizations. Extended lead times may necessitate larger safety stocks, increasing working capital requirements and potentially delaying timelines for sample processing in time-sensitive protocols. In parallel, any retaliatory measures or export controls can reduce the efficiency of international sample movement, complicating centralized testing models that rely on cross-border logistics and established cold-chain pathways.
Regulatory and contractual considerations also interact with tariff impacts; suppliers may seek to renegotiate service-level agreements or pass through cost increases, which can alter fixed-price trial budgets. To mitigate cumulative tariff effects, stakeholders may adopt strategies such as multi-sourcing, in-region manufacturing partnerships, and validated alternative assay platforms to preserve data integrity. Translational teams should monitor tariff developments closely, incorporate procurement flexibility into protocol risk registers, and align regulatory documentation to demonstrate assay continuity in the event of supplier or reagent substitutions.
Comprehensive segmentation-driven insights to align trial phase, study type, end-user capabilities, and therapeutic applications for optimized omics integration
A nuanced appreciation of trial segmentation is essential to align operational models with scientific objectives and regulatory expectations. Based on trial phase, studies span early first-in-human safety-focused investigations through late-stage confirmatory and post-market surveillance efforts, and each phase imposes distinct data quality, sample volume, and timeline requirements. Early-phase trials often prioritize rapid, hypothesis-generating molecular readouts with smaller cohorts, while later phases require scalable, validated assays and robust longitudinal sampling plans to support label-enabling claims.
Based on clinical trial type, study designs range from interventionally driven randomized and open-label models to observational architectures that capture natural history or real-world outcomes. Interventional trials incorporate modalities such as blinded or randomized controlled formats to minimize bias, whereas non-randomized and open-label approaches may be appropriate for mechanistic or rare-disease investigations where randomization is not feasible. Observational studies, including cohort, cross-sectional, prospective, and retrospective designs, provide vital context for biomarker prevalence, prognostic value, and real-world performance outside controlled experimental conditions.
Based on end user, the ecosystem includes academic and research institutes that combine exploratory science with investigator-led trials, contract research organizations that offer scalable operational throughput and regulatory experience, hospitals and diagnostic centers that enable clinical sample access and point-of-care integrations, and pharmaceutical and biotech companies that drive therapeutic intent and commercialization pathways. Each end user category contains further operational heterogeneity, from public and private academic centers to global and regional CROs, from diagnostic laboratories and private hospitals to large pharmaceutical enterprises and smaller biopharma innovators, necessitating tailored engagement models for sample logistics, data sharing agreements, and quality management.
Based on application, targeted therapeutic areas-cardiovascular, central nervous system, infectious, inflammatory, and oncology-present divergent biomarker landscapes and assay requirements. Cardiovascular programs demand precise phenotyping for arrhythmia, coronary disease, heart failure, and hypertension, while CNS studies require sensitive molecular and fluid biomarkers relevant to Alzheimer's, epilepsy, multiple sclerosis, and Parkinson's. Infectious disease research emphasizes pathogen detection and immune response profiling across hepatitis, HIV, influenza, and tuberculosis, whereas inflammatory disease trials focus on mechanistic markers in Crohn's disease, psoriasis, rheumatoid arthritis, and ulcerative colitis. Oncology efforts concentrate on tumor-specific genomic and proteomic signatures for breast, colorectal, lung, and prostate cancers. These segmentation axes should guide both scientific prioritization and the configuration of operational pipelines to ensure assay validity, regulatory readiness, and clinical relevance.
Regional operational and regulatory contours across the Americas, Europe, Middle East & Africa, and Asia-Pacific that determine trial feasibility and scientific representativeness
Regional dynamics materially shape operational design choices for omics-enabled trials, and each geography presents distinct advantages and constraints that sponsors must weigh when constructing global programs. In the Americas, strong translational research ecosystems, well-established biotechnology clusters, and deep clinical trial infrastructure create an environment conducive to complex multi-center studies and rapid assay validation. Regulatory pathways and payer dialogues in this region emphasize clinical utility and health economic demonstration, encouraging early incorporation of endpoints that can later support reimbursement discussions.
In Europe, Middle East & Africa, the regulatory landscape varies widely across jurisdictions, with several European agencies providing clear pathways for companion diagnostics and biomarker qualification while other markets require localized validation efforts. This diversity necessitates adaptable trial strategies, including region-specific analytical bridging studies, language and cultural considerations for patient-reported outcomes, and careful planning around cross-border sample movement. Capacity constraints in certain MEA markets can be mitigated through regional laboratory partnerships and investment in local training programs.
In Asia-Pacific, rapidly maturing clinical research infrastructure, high patient volumes, and increasing investment in precision medicine create compelling opportunities for accelerated enrollment and diverse genetic representation. However, operational teams must account for heterogeneity in regulatory timelines, data localization requirements, and differences in clinical practice patterns. Strategic collaborations with regional CROs and diagnostic laboratories, combined with early regulatory engagement, can unlock efficiencies and enhance the representativeness of molecular datasets across global development programs.
Understanding these regional contours enables sponsors to design hybrid trial footprints that leverage the strengths of each geography while proactively addressing logistical, regulatory, and ethical considerations that could influence data integrity and trial timelines.
Critical provider and sponsor competencies that determine successful deployment of omics assays and integrated operational models across clinical development
Key industry participants influencing omics-based trial execution include integrated sequencing and diagnostics providers, specialized bioinformatics firms, clinical laboratories, contract research organizations, academic translational centers, and pharmaceutical and biotechnology sponsors. Integrated sequencing and diagnostics providers play a pivotal role in scaling validated assays, maintaining reagent supply chains, and offering centralized data pipelines that support reproducible analyses across multi-site studies. Specialized bioinformatics organizations contribute standardized, regulatory-ready pipelines and data harmonization strategies that reduce analytic variability and accelerate interpretability.
Clinical laboratories and diagnostic centers serve as the operational backbone for sample receipt, processing, and storage, and their quality systems determine the reliability of molecular endpoints. Contract research organizations that have invested in omics capabilities bridge operational scale with regulatory expertise, enabling sponsors to outsource assay validation, centralized testing, and sample logistics under established quality agreements. Academic translational centers continue to drive innovation and early proof-of-concept studies, often partnering with industry to translate mechanistic insights into scalable clinical assumptions.
Pharmaceutical and biotech companies vary in their internal capabilities, with larger organizations more likely to internalize sequencing, bioinformatics, and regulatory teams, while smaller biopharma firms commonly rely on external partnerships to access specialized capabilities. The most influential players are those that successfully integrate cross-functional teams to align scientific hypothesis, assay validation, and commercial strategy, thereby reducing the friction between exploratory biomarker assessments and definitive clinical validation.
Actionable operational, procurement, and regulatory strategies that leaders should implement to reduce risk and accelerate omics-driven clinical development
Industry leaders should adopt an integrated approach that couples scientific rigor with operational flexibility to derive maximal value from omics-enabled trials. First, embed assay validation and data governance planning into protocol development so that analytical validation endpoints are defined early and harmonized across all participating sites. This preemptive alignment reduces downstream variability and accelerates regulatory conversations around biomarker utility.
Second, diversify procurement and supplier partnerships to mitigate single-source risk, especially for critical reagents, flow cells, and instrumentation that could be affected by trade policy or supply-chain disruptions. Prioritize vendors with regional manufacturing or distribution footprints to shorten lead times and improve contingency responsiveness. Third, invest in validated, reproducible bioinformatics pipelines and promote transparency in algorithmic decision-making to facilitate regulatory acceptance and cross-study comparability. Harmonized data formats and ontologies will accelerate meta-analyses and translational insight extraction.
Fourth, design trial footprints that balance centralized analytics with regional processing when necessary, using hybrid models to preserve sample integrity while accelerating enrollment and reducing logistical complexity. Fifth, cultivate collaborative agreements across academic centers, CROs, and diagnostic labs to share best practices, build capacity in under-resourced regions, and create standardized quality frameworks. Finally, maintain continuous engagement with regulatory authorities and payers to align evidence generation strategies with downstream market access expectations, ensuring that molecular endpoints are clinically meaningful and economically defensible.
Methodology combining expert interviews, operational mapping, and regulatory and protocol synthesis to derive actionable insights and pragmatic recommendations
The research methodology underpinning this analysis combines qualitative expert interviews, protocol and regulatory document reviews, and cross-disciplinary synthesis of operational practices and scientific literature. Expert interviews were conducted with clinical operations leaders, translational scientists, laboratory directors, and regulatory advisors to capture first-hand operational challenges, assay validation practices, and strategic responses to supply-chain pressures. Protocol and regulatory document reviews focused on public guidance, trial registries, and published companion diagnostic submissions to identify consistent evidence requirements and common validation milestones.
Operational practice analysis included mapping sample logistics, cold-chain management, laboratory accreditation standards, and contractual models between sponsors and external providers. Where publicly available, peer-reviewed studies and translational research publications were examined to corroborate assay performance characteristics and to extract lessons on cross-site comparability. The synthesis emphasizes reproducible procedures and regulatory-aligned documentation that sponsors can adopt to increase confidence in molecular endpoints.
Limitations inherent to the methodology include the dynamic nature of trade policy and reagent supply chains, and the variability of regional regulatory interpretations that can evolve rapidly. To mitigate these limitations, recommendations are framed to be adaptable, and stakeholders are encouraged to maintain direct regulatory dialogue and to revisit procurement strategies in light of the most current policy developments.
Synthesis of how disciplined operational execution and strategic planning convert omics innovation into clinically actionable evidence and sustainable development advantages
Omics-enabled clinical trials represent a pivotal shift in how therapeutic efficacy, safety, and mechanism are defined and measured. By integrating high-fidelity molecular data into trial design, sponsors can enhance patient selection, increase the interpretability of outcomes, and shorten timelines for meaningful go/no-go decisions when supported by robust operational infrastructure. The cumulative effect is a higher likelihood that mechanistic hypotheses translate into clinical insights that inform development strategy and commercialization planning.
However, the full promise of omics depends on disciplined operational execution: validated assays, resilient supply chains, reproducible analytics, and proactive regulatory engagement. Trade policy developments and regional heterogeneity introduce additional complexity that requires strategic procurement planning and flexible trial footprints. Ultimately, organizations that marry translational ambition with operational excellence will unlock more predictable pathways to regulatory acceptance and payer recognition, while also improving the scientific robustness of their clinical programs.
This analysis underscores the importance of early cross-functional alignment, diversified supplier strategy, and commitment to data standardization as the foundational elements for successful omics integration. Stakeholders who act on these principles position their programs to generate durable clinical evidence that supports both therapeutic differentiation and patient benefit.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Omics-Based Clinical Trials Market
Companies Mentioned
The key companies profiled in this Omics-Based Clinical Trials market report include:- Agilent Technologies, Inc.
- BioAro, Inc.
- BioNTech SE
- Bio‑Rad Laboratories, Inc.
- Bruker Corporation
- Charles River Laboratories International, Inc.
- Eli Lilly and Company
- Eurofins Scientific SE
- F. Hoffmann‑La Roche Ltd.
- Fulgent Genetics, Inc.
- GlaxoSmithKline plc
- ICON plc
- Illumina, Inc.
- IQVIA Holdings, Inc.
- Laboratory Corporation of America Holdings
- Novo Nordisk A/S
- Pacific Biosciences of California, Inc.
- Parexel International (MA) Corporation
- PerkinElmer, Inc.
- Pfizer, Inc.
- QIAGEN N.V.
- SGS Société Générale de Surveillance S.A.
- Signios Biosciences, Inc.
- Syneos Health, Inc.
- Thermo Fisher Scientific, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
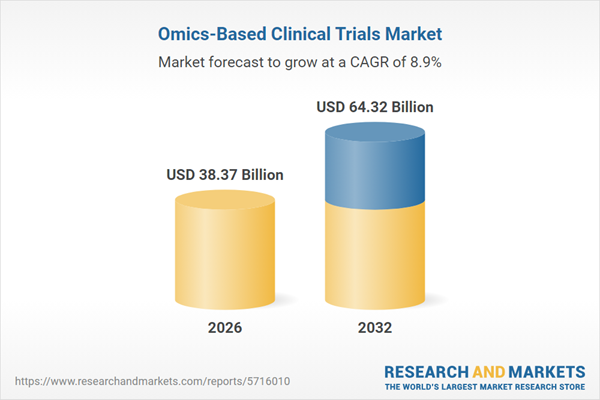
| Estimated Market Value ( USD | $ 38.37 Billion |
| Forecasted Market Value ( USD | $ 64.32 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |