Speak directly to the analyst to clarify any post sales queries you may have.
Strategic introduction to large volume nebulizers highlighting clinical utility, operational considerations, and evolving use cases across acute and chronic respiratory care
Large volume nebulizers have re-emerged as a critical component of respiratory care pathways across acute, subacute, and home settings as clinicians and providers seek reliable mechanisms for high-flow drug delivery. Unlike handheld nebulizer systems, large volume devices have distinct operational roles: they support continuous nebulization for patients requiring prolonged therapy, accommodate higher medication volumes, and integrate with oxygen and ventilatory circuits in both inpatient and ambulatory environments. These clinical and operational differentiators are central to procurement discussions, clinical protocols, and device selection criteria.
The introduction must also acknowledge how clinical practice patterns and patient demographics shape device utilisation. Aging populations and rising prevalence of chronic respiratory conditions have increased the demand for scalable delivery systems, while advances in drug formulations for inhalation therapy have expanded therapeutic possibilities. Consequently, stakeholders now balance clinical efficacy, ease of use, and lifecycle management when choosing between pneumatic, mesh, and ultrasonic platforms. Moving forward, the interplay between clinical evidence, device ergonomics, and service delivery models will determine which devices become standard fixtures across care pathways.
Assessment of transformative shifts reshaping the large volume nebulizer landscape including technology advancements, care delivery models, regulatory priorities, and supply chain dynamics
The landscape for large volume nebulizers is undergoing transformative shifts driven by technology maturation, evolving care delivery models, and heightened regulatory scrutiny. Technological advancements in mesh and ultrasonic platforms are improving aerosol particle size control and drug delivery efficiency, enabling more consistent dose administration and reducing treatment times. At the same time, improvements in device durability and integration capabilities are prompting clinicians to re-evaluate traditional preferences for jet-based systems, particularly in settings where noise, portability, and oxygen supplementation are operational concerns.
Concurrently, the rise of home-based care and outpatient treatment models is reshaping demand patterns, prompting manufacturers to prioritise user-friendly interfaces, simplified maintenance regimens, and compatibility with telehealth workflows. Regulatory frameworks are also evolving to place greater emphasis on device-human factors and post-market surveillance, which affects time-to-market and after-sales service models. As a result, supply chain resilience, vendor transparency, and lifecycle support are now equally as important as raw device performance metrics. Taken together, these forces are pushing the sector toward integrated solutions that balance clinical efficacy with pragmatic considerations around deployment, training, and long-term cost of ownership.
Evaluating the cumulative impact of United States tariffs in 2025 on procurement, manufacturing decisions, cost structures, and cross-border supply chains for nebulizer stakeholders
The imposition of United States tariffs in 2025 has generated downstream effects that require careful strategic navigation across manufacturing footprints, procurement strategies, and international supply chains. Tariff measures have effectively altered the calculus for manufacturers that rely on component imports, prompting reassessments of sourcing, inventory buffers, and contractual terms with suppliers. In response, many organisations have accelerated nearshoring and diversification efforts to reduce exposure to tariff volatility, while others have renegotiated long-term supply agreements to lock in price stability and guarantee component flows.
These shifts have further implications for buyers and clinical systems. Hospitals and distributors are now more closely scrutinising total landed costs and warranty structures rather than focusing solely on upfront device pricing. For some organisations, the tariffs have accelerated the adoption of local manufacturing partnerships and contract manufacturing arrangements that absorb a greater share of compliance and logistics risk. Meanwhile, innovation cycles can be affected when manufacturers redirect capital to supply-chain reconfiguration rather than rapid product development. Overall, the cumulative impact of tariffs has emphasised the need for resilient procurement strategies, transparent supplier relationships, and adaptive sourcing models that preserve clinical continuity while mitigating cost and delivery risks.
In-depth segmentation insights revealing product, application, end user, and distribution channel dynamics that define adoption patterns and purchasing behaviour across care settings
Product, application, end user, and distribution dynamics together shape adoption patterns and purchasing behaviour for large volume nebulizer solutions. From the product standpoint, stakeholders evaluate jet nebulizers, mesh nebulizers, and ultrasonic nebulizers across a range of clinical and operational criteria. Jet nebulizers include pneumatic jet and venturi jet subtypes that maintain relevance in high-flow and oxygen-integrated applications, while mesh nebulizers are differentiated between static mesh and vibrating mesh variations that favour quiet operation and improved particle size control. Ultrasonic nebulizers, in turn, are categorised by high frequency and low frequency configurations that influence aerosol generation efficiency and compatibility with specific drug formulations.
Clinical applications such as asthma, bronchiectasis, COPD, and cystic fibrosis drive product selection through distinct therapeutic needs and dosing regimens, and differences in disease progression mandate variable treatment durations and device resilience. End users span ambulatory care centres, clinics, home care settings, and hospitals, each with tailored operational requirements. Ambulatory care centres include daycare surgery centres and outpatient treatment centres where throughput and infection control are priorities; clinics encompass general practice and respiratory specialty settings that prioritise ease of use and maintenance; home care settings include home health agencies and personal use scenarios where portability, user training, and remote monitoring matter most; and hospitals range from community health centres to tertiary care centres where integration with complex respiratory circuits and robustness under continuous use are critical. Distribution channels also shape market access and fulfilment strategies, with direct sales occurring via original equipment manufacturers and third-party distributors, hospital pharmacies divided into private and public units, online channels spanning e-commerce marketplaces and manufacturer websites, and retail pharmacies represented by chain and independent outlets that influence point-of-care stocking and replacement part availability.
Taken together, these segmentation layers inform where manufacturers invest in product differentiation, where service providers allocate training resources, and how procurement teams prioritise contract terms and after-sales support. This granular view helps stakeholders align device features to clinical workflows and optimise distribution touchpoints to reduce downtime and improve patient outcomes.
Regional analysis synthesising demand drivers, clinical infrastructure, reimbursement environments, and adoption hurdles across the Americas, Europe Middle East Africa and Asia-Pacific
Regional dynamics exert a profound influence on adoption drivers, reimbursement mechanisms, regulatory burdens, and operational readiness for large volume nebulizer deployment. In the Americas, capital investment patterns and established hospital networks tend to favour device standardisation and group purchasing arrangements, which heighten the importance of robust clinical evidence and long-term service agreements. Europe, Middle East & Africa presents a heterogeneous regulatory and reimbursement landscape where country-level policies and procurement practices create both barriers and pockets of opportunity, particularly for manufacturers that can offer regulatory support and adaptable distribution models. Asia-Pacific combines rapid infrastructure investment with diverse care delivery models, where public hospital procurement, growing private care segments, and heightened focus on home healthcare services create complex demand signals.
These regional differences translate into practical implications for manufacturers and buyers. For instance, supply-chain design and inventory strategies must reflect regional lead times and regulatory approval cycles, while sales and clinical engagement strategies require localisation of training materials and evidence dossiers. Moreover, reimbursement approaches and purchasing preferences vary significantly, requiring nimble commercial models that balance direct sales, distributor partnerships, and digital channels. Ultimately, successful regional strategies align product portfolios with local clinical priorities, regulatory expectations, and channel dynamics to accelerate adoption and reduce friction during procurement and deployment.
Key company strategies and competitive dynamics for manufacturers, suppliers, and service providers focused on product innovation, partnerships, and global market access pathways
Competitive dynamics in the large volume nebulizer space are defined by a mix of legacy manufacturers, specialised device innovators, contract manufacturers, and channel partners who compete on product performance, after-sales support, and regulatory compliance. Leading companies strategically differentiate by demonstrating clinical outcomes, streamlining device maintenance, and offering integrated service packages that include training and remote monitoring capabilities. Partnerships with pharmaceutical companies developing inhalation therapies have become an increasingly important route to co-development and co-marketing, as device-drug combinations require tighter clinical alignment and regulatory coordination.
Investment in manufacturing capacity and quality systems remains a core competitive lever, particularly when regulatory authorities expect robust post-market surveillance and traceability. Companies that have diversified manufacturing footprints or established local assembly operations are better positioned to mitigate tariff and logistics disruptions. At the same time, digital enablement-ranging from connectivity for adherence monitoring to cloud-based analytics for device performance-creates opportunities for new entrants to capture share by offering value-added services. In summary, competitive success hinges on combining proven device performance with scalable manufacturing, regulatory fluency, and service-led commercial models that reduce end-user friction and support long-term clinical adoption.
Actionable recommendations for industry leaders to optimise product portfolios, distribution models, regulatory engagement, and value-based care integration for nebulizer solutions
Industry leaders should prioritise a set of pragmatic actions to strengthen resilience, accelerate adoption, and capture strategic opportunities across clinical settings. First, diversify supply chains and consider regional assembly or contract manufacturing arrangements to reduce exposure to tariff and logistics volatility. Second, invest in device interoperability and human-factor design to improve clinician acceptance and patient adherence, thereby shortening procurement approval cycles and increasing utilisation across care pathways. Third, establish formal partnerships with pharmaceutical developers and clinical networks to co-design drug-device combinations and generate the clinical evidence required for guideline adoption.
Additionally, leaders should adopt hybrid commercial models that blend direct sales for institutional customers with expanded digital channels and distributor networks to reach home care markets efficiently. Regulatory engagement must be proactive: engage with authorities early in product development, plan for robust post-market surveillance, and prepare localised compliance documentation to ease market entry. Finally, align commercial incentives around service and outcomes by offering bundled maintenance, training, and analytics services that demonstrate lifecycle value. By executing these steps in coordinated fashion, organisations will reduce procurement friction, protect margins, and position their portfolios for sustainable adoption.
Transparent research methodology detailing primary and secondary approaches, validation steps, regulatory parsing, and quality assurance measures underpinning report findings
The research underpinning this analysis employs a mixed-methods approach that combines structured primary interviews with clinicians, procurement officers, and device engineers, alongside comprehensive secondary review of regulatory guidance, clinical literature, and supply chain data. Primary engagements were designed to capture operational priorities, real-world performance trade-offs, and procurement decision drivers across acute, ambulatory, and home care settings. Secondary analysis synthesised device specifications, regulatory filings, and published clinical outcomes to validate interview insights and identify cross-cutting themes.
To ensure rigor, findings were triangulated through cross-validation with manufacturing and distribution stakeholders, and key assumptions were stress-tested via scenario analysis that considered variations in supply chain disruptions and regulatory timelines. Quality assurance procedures included peer reviews by subject-matter experts in respiratory therapy and medical device regulation, and a documented audit trail of sources and interview protocols. Limitations include variation in regional regulatory detail and evolving tariff policies, which were explicitly noted and accounted for during synthesis to preserve transparency and contextual fidelity.
Concise conclusion synthesising strategic takeaways, emergent risks and opportunities, and next‑step considerations for stakeholders in the large volume nebulizer ecosystem
In conclusion, the large volume nebulizer sector is at an inflection point where technological evolution, shifting care delivery models, and geopolitical trade dynamics intersect to redefine strategic priorities. Stakeholders that proactively align product capabilities with clinical workflows, diversify sourcing strategies, and invest in regulatory and service infrastructures will be best positioned to benefit from sustained adoption across inpatient, ambulatory, and home settings. Conversely, organisations that under-estimate the operational complexity of device support and supply-chain resilience risk procurement pushback and slower uptake.
The path forward requires disciplined cross-functional collaboration among clinical, procurement, regulatory, and commercial teams to translate insight into executable plans. Stakeholders should focus on demonstrable clinical benefits, scalable service models, and adaptable supply-chain architectures. By doing so, they will reduce time-to-adoption, mitigate exposure to policy shifts, and capture the long-term value associated with reliable, high-volume aerosol delivery solutions.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
16. China Large Volume Nebulizer Market
Companies Mentioned
The key companies profiled in this Large Volume Nebulizer market report include:- Aeroflow Healthcare
- ATOM Medical Corporation
- B&B Medical Technologies
- Besco Medical Co. Ltd.
- Briggs Healthcare
- Flaem Nuova S.p.A.
- GaleMed Corporation
- GF Health Products Inc.
- Heyer Medical AG
- La Diffusion Technique Française
- Mabis Healthcare Inc.
- Medquip Inc.
- Nidek Medical Products Inc.
- Omron Healthcare Co. Ltd.
- PARI GmbH
- R.S. Medical Inc.
- Rossmax International Ltd.
- Salter Labs
- Teleflex Medical
- Trudell Medical International
- VORTRAN Medical Technology
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
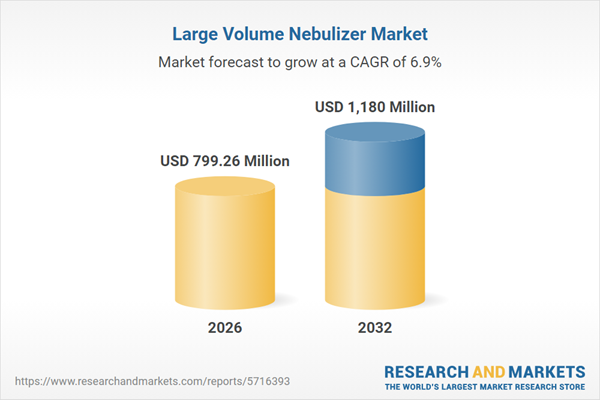
| Estimated Market Value ( USD | $ 799.26 Million |
| Forecasted Market Value ( USD | $ 1180 Million |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |