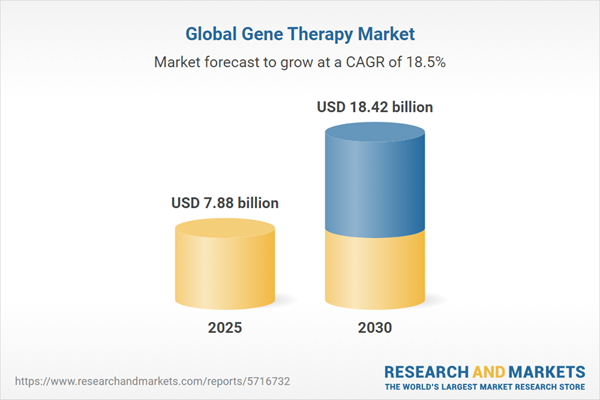
The global gene therapy market is experiencing robust growth from 2025 to 2030, driven by increased investment in research and development (R&D) and the rising prevalence of diseases like cancer. Gene therapy, which involves modifying or replacing defective genes to treat or prevent diseases, is gaining traction due to its potential to address complex conditions such as cancer and rare genetic disorders. The market is propelled by advancements in immunotherapy, favorable regulatory environments, and growing funding for innovative treatments. The United States leads the market, supported by significant R&D investments and a high incidence of cancer. However, high treatment costs and regulatory complexities may pose challenges.
Market Drivers
Rising Prevalence of Cancer
The increasing incidence of cancer is a primary driver of the gene therapy market. In the United States, cancer cases rose to approximately 1.92 million in 2022, with 609,000 deaths, underscoring the urgent need for innovative treatments. Gene therapies, particularly those targeting advanced-stage tumors through immunotherapy, are gaining importance for their ability to improve remission rates. The growing patient pool drives demand for therapies like chimeric antigen receptor T-cell (CAR-T) and vector-based treatments, which offer targeted solutions for various cancer types.Increased R&D Investment
Significant investments in gene therapy R&D are fueling market growth. In 2023, the World Health Organization reported that global healthcare R&D spending, including for gene therapy, continues to rise, with Western Pacific countries leading at 0.07% of GDP. In the United States, the National Institutes of Health (NIH) allocated substantial funding for gene therapy research, fostering innovation in treatments for cancer and rare diseases. These investments support the development of advanced therapies, such as oncolytic virotherapy and vector-based treatments, enhancing market penetration.Favorable Regulatory Environment
The regulatory landscape, particularly in the United States, supports market growth. The FDA's approval of therapies like Vyjuvek in May 2023 for dystrophic epidermolysis bullosa and Adstiladrin in December 2022 for non-muscle-invasive bladder cancer demonstrates a commitment to advancing gene therapy. These approvals, coupled with streamlined regulatory pathways, encourage innovation and market entry, boosting the adoption of gene therapies for various indications.Market Restraints
The gene therapy market faces challenges due to the high cost of treatments, often reaching millions of dollars per patient, which limits accessibility, particularly in developing regions. Regulatory complexities, including stringent safety and efficacy requirements, can delay approvals and increase development costs. Additionally, the complexity of manufacturing gene therapies, such as viral vectors, poses scalability challenges. Addressing these barriers through cost optimization and standardized production processes will be critical for sustained market growth.Market Segmentation
By Application
The market is segmented into oncology, rare genetic disorders, neurological disorders, and others. Oncology dominates due to the high prevalence of cancer and the focus on immunotherapies like CAR-T and oncolytic virotherapy. Rare genetic disorders, such as Duchenne muscular dystrophy, are also significant, driven by therapies like RGX-202.By Geography
The market is segmented into North America, Europe, Asia-Pacific, South America, and the Middle East and Africa. North America, led by the United States, holds a dominant share due to its high cancer incidence, substantial R&D funding, and favorable regulatory environment. Asia-Pacific is expected to grow rapidly, driven by increasing healthcare investments and rising disease prevalence in countries like India. Europe, South America, and the Middle East and Africa are emerging markets, supported by improving healthcare infrastructure.The gene therapy market is set for robust growth from 2025 to 2030, driven by rising cancer prevalence, increased R&D investment, and favorable regulations, particularly in the United States. Despite challenges from high costs and regulatory complexities, the market's outlook remains positive, with strong growth in North America and Asia-Pacific. Industry players must focus on cost-effective therapies, scalable manufacturing, and strategic collaborations to capitalize on the growing demand for innovative gene therapy solutions.
Key Benefits of this Report:
- Insightful Analysis: Gain detailed market insights covering major as well as emerging geographical regions, focusing on customer segments, government policies and socio-economic factors, consumer preferences, industry verticals, and other sub-segments.
- Competitive Landscape: Understand the strategic maneuvers employed by key players globally to understand possible market penetration with the correct strategy.
- Market Drivers & Future Trends: Explore the dynamic factors and pivotal market trends and how they will shape future market developments.
- Actionable Recommendations: Utilize the insights to exercise strategic decisions to uncover new business streams and revenues in a dynamic environment.
- Caters to a Wide Audience: Beneficial and cost-effective for startups, research institutions, consultants, SMEs, and large enterprises.
What do businesses use our reports for?
Industry and Market Insights, Opportunity Assessment, Product Demand Forecasting, Market Entry Strategy, Geographical Expansion, Capital Investment Decisions, Regulatory Framework & Implications, New Product Development, Competitive Intelligence.Report Coverage:
- Historical data from 2020 to 2024 & forecast data from 2025 to 2030
- Growth Opportunities, Challenges, Supply Chain Outlook, Regulatory Framework, and Trend Analysis
- Competitive Positioning, Strategies, and Market Share Analysis
- Revenue Growth and Forecast Assessment of segments and regions including countries
- Company Profiling: Strategies, Products, Financial Information, and Key Developments among others
Segmentation:
By Therapy Type
- Ex Vivo
- In Vivo
- In Situ
By Vector Type
- Viral
- Non-Viral
By Technique Type
- Gene Augmentation
- Gene Inhibition
- Specific Cell Kill
By Disease Type
- Cancer
- Heart Disease
- Diabetes
- HIV
- Others
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- France
- United Kingdom
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- India
- Japan
- South Korea
- Indonesia
- Thailand
- Others
Table of Contents
Companies Mentioned
- Shanghai Sunway Biotech Co. Ltd
- Pfizer In
- Astellas Gene Therapies, Inc.
- Novartis Gene Therapies
- MeiraGTx
- San Rocco Therapeutics
- American Gene Technologies
- Lysogene
- AVROBIO Inc
- Adverum Biotechnologies Inc
- Spark Therapeutics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 152 |
| Published | August 2025 |
| Forecast Period | 2025 - 2030 |
| Estimated Market Value ( USD | $ 7.88 billion |
| Forecasted Market Value ( USD | $ 18.42 billion |
| Compound Annual Growth Rate | 18.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |