Expanding Applications in Biopharmaceutical Processing Drive Europe Medical Tubing Market
The utilization of medical tubing is on the rise in the pharmaceutical and biopharmaceutical industries. Bioprocessing involves utilizing living cells or their components to produce desired products. This process requires specialized tubing solutions to ensure efficiency, safety, and adherence to strict regulatory standards. The purity of materials used is crucial in bioprocessing applications.Medical-grade tubing made from biocompatible materials such as silicone and thermoplastic elastomers (TPE) is essential to prevent the leaching of harmful substances into biopharmaceutical products. The increasing regulatory emphasis on product safety and efficacy drives the demand for medical tubing solutions that guarantee high levels of purity and biocompatibility during bioprocessing.
Various market players are launching medical-grade tubing with applications in biopharmaceutical fluid processing. In October 2023, RAUMEDIC expanded its range of biopharmaceutical fluid processing products by introducing a new brand of biocompatible tubing. Its portfolio includes a diverse selection of biocompatible fluid processing tubing systems - SILMOTION (silicone tubing), BRAIDMOTION (braided silicone tubing), THERMMOTION (thermoplastic elastomer tubing), PVC tubing, and FEP tubing.
Bioprocessing frequently involves transporting sensitive biological materials, necessitating specialized tubing that can withstand varying pressures and flow rates without compromising the integrity of the contents. Advanced medical tubing is also utilized for fluid transport in bioprocessing applications, improving the efficiency of biopharmaceutical manufacturing processes. In October 2021, DuPont introduced a new range of extruded pharmaceutical tubing made from TPE. The newly launched Liveo Pharma TPE Tubing products are designed for fluid transport and single-use bioprocessing applications, maintaining the high standards of the Liveo Silicone Pharma Tubing lines.
The TPE tubing range of DuPont complements its existing silicone products while prioritizing quality, purity, and performance. Its benefits include improved heat welding, high tensile strength, burst resistance, minimal spallation after pumping, suitability for low extractable volumes, and good chemical resistance. This tubing is ideal for biopharma processing, enabling aseptic connections without the need for connectors; it is also suitable for peristaltic pump applications. Thus, expanding applications of medical tubing in bioprocessing, and fluid transport and handling applications propels the Europe medical tubing market .
Europe Medical Tubing Market Overview
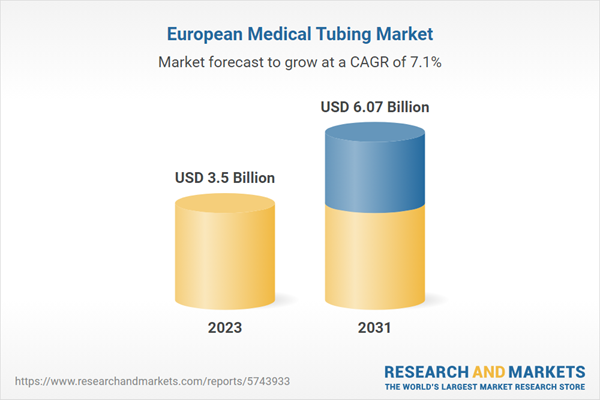
The Europe medical tubing market is segmented into the UK, Germany, France, Italy Spain, and the Rest of Europe. Strategic development by key players, the surge in patients with chronic diseases, and government initiatives to improve healthcare infrastructure are anticipated to drive market growth. Switzerland, Belgium, Greece, Norway, Portugal, Sweden, the Czech Republic, Croatia, Ireland, Denmark, the Netherlands, and Russia are among the key countries in the Rest of Europe medical tubing market. The emphasis on product innovations, quality, and product differentiation while developing healthcare products enhances the market's growth in Switzerland, Belgium, Greece, Norway, and Denmark. Focus on innovation and quality in Switzerland encourages developments in medical tubing solutions in high-tech applications.Europe Medical Tubing Market Revenue and Forecast to 2031 (US$ Million)
Europe Medical Tubing Market Segmentation
The Europe medical tubing market is categorized into type, structure, application, end users, and country.- By type, the Europe medical tubing market is segmented into Polyvinyl Chloride (PVC), polyimide or nylons, PTFE or thermoplastic elastomers (TPES), thermoplastic polyurethanes (TPUS), polyvinylidene Fluoride (PVDF), polypropylene and polyethylene, silicone, and others. The Polyvinyl Chloride (PVC) segment held the largest share of the Europe medical tubing market share in 2023.
- In terms of structure, the Europe medical tubing market is segmented into single-lumen, multi-lumen, multi-layer extruded tubing, tapered or bump tubing, braided tubing, balloon tubing, corrugated tubing, heat shrink tubing, and others. The multi-layer extruded tubing segment held the largest share of the Europe medical tubing market share in 2023.
- Based on application, the Europe medical tubing market is segmented into bulk disposable tubing, catheters and cannula, drug delivery systems, and others. The bulk disposable tubing segment held the largest share of the Europe medical tubing market share in 2023.
- By end users, the Europe medical tubing market is segmented into hospital and clinics, ambulatory care centers, medical labs, and others. The hospital and clinics segment held the largest share of the Europe medical tubing market share in 2023.
- Based on country, the Europe medical tubing market is segmented into Germany, France, the UK, Italy, Spain, and the Rest of Europe. The Rest of Europe segment held the largest share of Europe medical tubing market in 2023.
Reasons to Buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Europe medical tubing market.
- Highlights key business priorities to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the Europe medical tubing market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth Europe market trends and outlook coupled with the factors driving the Europe medical tubing market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution.
Table of Contents
Companies Mentioned
Some of the leading companies in the Europe Medical Tubing Market include:- Accu-Tube LLC
- Axiom Medical Inc
- Boston Scientific Corp
- Compagnie de Saint Gobain SA
- DuPont de Nemours Inc
- Freudenberg Medical LLC
- MicroLumen Inc
- Nordson Corp
- Polyzen Inc
- Raumedic AG
- TE Connectivity Ltd
- TEKNI-PLEX
- Teleflex Inc
- The Lubrizol Corporation
- Trelleborg AB
- W L Gore and Associates Inc
- Zeus Industrial Products Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 223 |
| Published | March 2025 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 3.5 Billion |
| Forecasted Market Value ( USD | $ 6.07 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 18 |