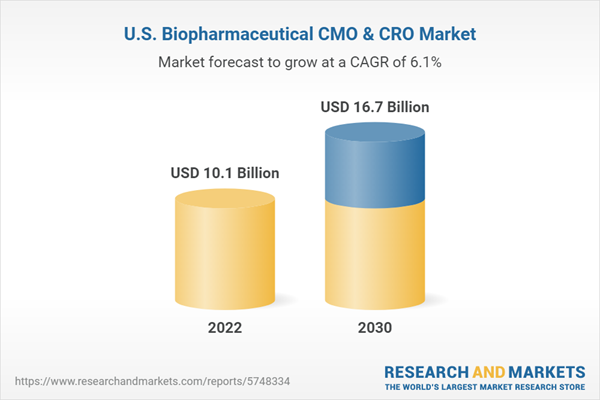
The U.S. biopharmaceutical CMO & CRO market size is expected to reach USD 16.7 billion by 2030, expanding at a CAGR of 6.1% during the forecast period. The contract manufacturing organization (CMO) and contract research organization (CRO) industries have seen significant growth in recent years, particularly in the U.S. This growth is driven by a variety of factors, including the increasing demand for biologics and other complex therapies, the need for more flexible and scalable manufacturing capabilities, and the desire to reduce supply chain risks and costs.
Another factor driving the expansion of CMO and CRO manufacturing facilities in the U.S. is the need for more flexible and scalable manufacturing capabilities. The biopharmaceutical industry is characterized by rapid technological change and changing demands, which can create challenges for companies that have invested heavily in fixed manufacturing assets. By outsourcing to CMOs and CROs, pharmaceutical companies can access manufacturing and research capacity on an as-needed basis, without incurring the fixed costs associated with building and maintaining their own facilities. This approach allows companies to more quickly and efficiently respond to changes in market demand.
The increased demand for biopharmaceutical research and manufacturing has resulted in CMOs and CROs acquiring existing biopharmaceutical manufacturing and research facilities or building new facilities in their existing plants. This is because the expansion of these facilities increases the capacity to meet the growing demand for biologics and other biopharmaceutical products.
With the U.S. being a major market for biopharmaceuticals, expansion of facilities in this region is crucial for companies to cater to the needs of their customers and stay competitive in the market. For instance, FUJIFILM Diosynth Biotechnologies announced plans to expand their Taxes plant by adding 138,000 sq feet by 2024. This expansion will lead to the construction of a new cGMP production facility that is expected to become operational in 2024. This expansion is expected to double the advanced therapy and vaccine manufacturing capacity of the company in the U.S.
This product will be delivered within 1-3 business days.
Another factor driving the expansion of CMO and CRO manufacturing facilities in the U.S. is the need for more flexible and scalable manufacturing capabilities. The biopharmaceutical industry is characterized by rapid technological change and changing demands, which can create challenges for companies that have invested heavily in fixed manufacturing assets. By outsourcing to CMOs and CROs, pharmaceutical companies can access manufacturing and research capacity on an as-needed basis, without incurring the fixed costs associated with building and maintaining their own facilities. This approach allows companies to more quickly and efficiently respond to changes in market demand.
The increased demand for biopharmaceutical research and manufacturing has resulted in CMOs and CROs acquiring existing biopharmaceutical manufacturing and research facilities or building new facilities in their existing plants. This is because the expansion of these facilities increases the capacity to meet the growing demand for biologics and other biopharmaceutical products.
With the U.S. being a major market for biopharmaceuticals, expansion of facilities in this region is crucial for companies to cater to the needs of their customers and stay competitive in the market. For instance, FUJIFILM Diosynth Biotechnologies announced plans to expand their Taxes plant by adding 138,000 sq feet by 2024. This expansion will lead to the construction of a new cGMP production facility that is expected to become operational in 2024. This expansion is expected to double the advanced therapy and vaccine manufacturing capacity of the company in the U.S.
U.S. Biopharmaceutical CMO & CRO Market Report Highlights
- Mammalian cell line-based bioproduction system is the largest segment and is expected to grow at a CAGR of 6.3% during the forecast period.
- The contract research service types segment is expected to register the fastest CAGR of 6.9% over the forecast period
- The biologics product segment accounted for the largest market share of 82.4% in the year 2022
- This can be attributed to the local presence of several contract research service providers in the country. The U.S. has a higher concentration and number of biopharmaceutical CMOs and CROs in comparison with other countries
This product will be delivered within 1-3 business days.
Table of Contents
Chapter 1 Research Methodology1.1 Market Segmentation & Scope
1.2 Market Definition
1.3 Information Procurement
1.3.1 Purchased database
1.3.2 internal database
1.3.3 Secondary sources & third party perspectives
1.3.4 Primary research
1.4 Information Analysis
1.4.1 Data analysis models
1.5 Market Formulation & Data Visualization
1.6 Data Validation & Publishing
Chapter 2 Executive Summary
2.1 Biopharmaceutical CMO & CRO Market Outlook, 2018 - 2030
Chapter 3 Industry Outlook: Market Variables, Trends, & Scope
3.1 Penetration &Growth Prospect Mapping For Contract Manufacturing Service, 2018
3.2 Trend Analysis
3.2.1 Source trend
3.2.2 Service trend
3.2.3 Product trend
3.2.4 Regional trend
3.3 Biopharmaceutical CMO & CRO Market: Market Dynamics
3.3.1 Market driver analysis
3.3.1.1 Rising investment by CMOs for capacity expansion
3.3.1.2 Commercial success of biopharmaceuticals and consequent increased demand for the biopharmaceuticals
3.3.1.3 Robust biopharmaceuticals pipeline
3.3.1.4 Cost and time-saving benefits offered by contract services
3.3.2 Market restraint analysis
3.3.2.1 Limited outsourcing among well-established biopharmaceutical manufacturers
3.4 Key Opportunity Analysis
3.4.1 Rising funds in the biopharmaceutical industry
3.4.2 Mergers & acquisition for facility expansion
3.4.3 Growing demand for protein therapeutics
3.5 Industry Analysis - Porter’s
3.5.1 Supplier Power: Substantial number of suppliers for bioprocessing equipment contributes to the low supplier power
3.5.2 Buyer Power: Fragmented nature of the biopharmaceutical industry has led to the moderate bargaining power of buyers
3.5.3 Substitution Threat: High due to the presence of several big pharma companies
3.5.4 New Entrants Threat: Presence of established players coupled with the need for adequate capacity results in a moderate threat of new entrants
3.5.5 Competitive Rivalry: High due to the fragmented nature of the market
3.6 Biopharmaceuticals CMO & CRO-SWOT Analysis, By PEST
3.6.1 Political landscape
3.6.2 Economic landscape
3.6.3 Social landscape
3.6.4 Technology landscape
3.7 COVID-19 Impact and Reformation Analysis
Chapter 4 U.S. Biopharmaceuticals CMO & CRO Market: Source Estimates & Trend Analysis
4.1 U.S. Biopharmaceuticals CMO & CRO Market: Source Movement Analysis
4.2 Mammalian Source
4.2.1 U.S. mammalian source market, 2018 - 2030 (USD Million)
4.3 Non-mammalian Source
4.3.1 U.S. mammalian source market, 2018 - 2030 (USD Million)
Chapter 5 U.S. Biopharmaceuticals CMO & CRO Market: Service Type Estimates & Trend Analysis
5.1 U.S. Biopharmaceuticals CMO & CRO Market: Service Type Movement Analysis
5.2 Contract Manufacturing, by service
5.2.1 U.S. contract manufacturing market, 2018 - 2030 (USD Million)
5.2.2 Process development
5.2.2.1 U.S. process development market, 2018 - 2030 (USD Million)
5.2.2.2 Downstream processing
5.2.2.2.1 U.S. downstream processing market, 2018 - 2030 (USD Million)
5.2.2.3 Upstream processing
5.2.2.3.1 U.S. upstream processing market, 2018 - 2030 (USD Million)
5.2.3 Fill & finish operations
5.2.3.1 U.S. fill & finish operations market, 2018 - 2030 (USD Million)
5.2.4 Analytical & QC testing
5.2.4.1 U.S. analytical & QC testing market, 2018 - 2030 (USD Million)
5.2.5 Packaging
5.2.5.1 U.S. contract packaging market, 2018 - 2030 (USD Million)
5.3 Contract Research
5.3.1 U.S. contract research market, 2018 - 2030 (USD Million)
5.3.2 Oncology
5.3.2.1 U.S. oncology market, 2018 - 2030 (USD Million)
5.3.3 Inflammation & immunology
5.3.3.1 U.S. inflammation & immunology market, 2018 - 2030 (USD Million)
5.3.4 Cardiology
5.3.4.1 U.S. cardiology market, 2018 - 2030 (USD Million)
5.3.5 Neuroscience
5.3.5.1 U.S. neuroscience market, 2018 - 2030 (USD Million)
5.3.6 Others
5.3.6.1 U.S. other CRO services market, 2018 - 2030 (USD Million)
Chapter 6 U.S. Biopharmaceuticals CMO & CRO Market: Product Estimates & Trend Analysis
6.1 U.S. Biopharmaceuticals CMO & CRO Market: Product Movement Analysis
6.2 Biologics
6.2.1 U.S. biologics market, 2018 - 2030 (USD Million)
6.2.2 Monoclonal Antibodies
6.2.2.1 U.S. monoclonal antibodies market, 2018 - 2030 (USD Million)
6.2.3 Recombinant proteins
6.2.3.1 U.S. recombinant proteins market, 2018 - 2030 (USD Million)
6.2.4 Vaccines
6.2.4.1 U.S. vaccines market, 2018 - 2030 (USD Million)
6.2.5 Antisense, RNAi, & molecular therapy
6.2.5.1 U.S. antisense, RNAi, &molecular therapy market, 2018 - 2030 (USD Million)
6.2.6 Others
6.2.6.1 U.S. other product market, 2018 - 2030 (USD Million)
6.3 Biosimilars
6.3.1 U.S. biosimilars market, 2018 - 2030 (USD Million)
Chapter 7 Competitive Landscape
7.1 Company Profiles
7.1.1 Boehringer Ingelheim GmbH
7.1.1.1 Company overview
7.1.1.2 Financial performance
7.1.1.3 Product benchmarking
7.1.1.4 Strategic initiatives
7.1.2 LONZA
7.1.2.1 Company overview
7.1.2.2 Financial performance
7.1.2.3 Product benchmarking
7.1.2.4 Strategic initiatives
7.1.3 Catalent
7.1.3.1 Company overview
7.1.3.2 Financial performance
7.1.3.3 Product benchmarking
7.1.3.4 Strategic initiatives
7.1.4 Rentschler Biotechnologie GmbH
7.1.4.1 Company overview
7.1.4.2 Financial performance
7.1.4.3 Product benchmarking
7.1.4.4 Strategic initiatives
7.1.5 Thermo Fisher Scientific Inc
7.1.5.1 Company overview
7.1.5.2 Financial performance
7.1.5.3 Product benchmarking
7.1.5.4 Strategic initiatives
7.1.6 AGC Biologics
7.1.6.1 Company overview
7.1.6.2 Financial performance
7.1.6.3 Product benchmarking
7.1.6.4 Strategic initiatives
7.1.7 Fujifilm Diosynth Biotechnologies
7.1.7.1 Company overview
7.1.7.2 Financial performance
7.1.7.3 Product benchmarking
7.1.7.4 Strategic initiatives
7.1.8 Abzena
7.1.8.1 Company overview
7.1.8.2 Financial performance
7.1.8.3 Product benchmarking
7.1.8.4 Strategic initiatives
7.1.9 Samsung Biologics
7.1.9.1 Company overview
7.1.9.2 Financial performance
7.1.9.3 Product benchmarking
7.1.9.4 Strategic initiatives
7.1.10 WuXi Biologics
7.1.10.1 Company overview
7.1.10.2 Financial performance
7.1.10.3 Product benchmarking
7.1.10.4 Strategic initiatives
7.1.11 AbbVie Inc.
7.1.11.1 Company overview
7.1.11.1.1 DPx
7.1.11.2 Financial performance
7.1.11.3 Product benchmarking
7.1.11.4 Strategic initiatives
7.1.12 Charles River Laboratories International, Inc.
7.1.12.1 Company overview
7.1.12.2 Financial performance
7.1.12.3 Product benchmarking
7.1.12.4 Strategic initiatives
7.1.13 ICON Plc
7.1.13.1 Company overview
7.1.13.2 Financial performance
7.1.13.3 Product benchmarking
7.1.13.4 Strategic initiatives
7.1.14 Parexel International Corporation
7.1.14.1 Company overview
7.1.14.2 Financial performance
7.1.14.3 Product benchmarking
7.1.14.4 Strategic initiatives
7.1.15 Labcorp.
7.1.15.1 Company overview
7.1.15.2 Financial performance
7.1.15.3 Product benchmarking
7.1.15.4 Strategic initiatives
List of Tables
Table 1 2020 pipeline of biologics
TABLE 2 Some commercially available biopharmaceuticals and biologics produced using both mammalian & non-mammalian cell lines
TABLE 3 Some commercially available biopharmaceuticals and biologics produced using only mammalian cell lines
TABLE 4 Some biopharmaceuticals produced using S. cerevisiae
TABLE 5 Some commercially available biopharmaceuticals and biologics produced using only microbial cell lines
TABLE 6 Some commercially available monoclonal antibody products
TABLE 7 Number of MAbs products by indication area, 2015
TABLE 8 Time and budget requirements for different stages of vaccine development
List of Figures
Fig. 1 Market research process
FIG. 2 Information Procurement
FIG. 3 Primary research pattern
FIG. 4 Market research approaches
FIG. 5 Value chain-based sizing & forecasting
FIG. 6 QFD modeling for market share assessment
FIG. 7 Market segmentation & scope
FIG. 8 Biopharmaceutical CMO & CRO market outlook, 2018 - 2030
FIG. 9 Market trends & outlook
FIG. 10 Market driver relevance analysis (Current & future impact)
FIG. 11 Number of Reference Products by U.S. Biosimilars Launchable Dates
FIG. 12 Market restraint relevance analysis (Current & future impact)
FIG. 13 Risk associated with outsourcing services
FIG. 14 SWOT Analysis, By Factor (political & legal, economic and technological)
FIG. 15 Porter’s Five Forces Analysis
FIG. 16 Strategy framework
FIG. 17 U.S. biopharmaceuticals CMO & CRO market: Source outlook key takeaways
FIG. 18 U.S. Biopharmaceuticals CMO & CRO market: Source movement analysis
FIG. 19 U.S. mammalian source market, 2018 - 2030 (USD Million)
FIG. 20 U.S. non-mammalian source market, 2018 - 2030 (USD Million)
FIG. 21 U.S. biopharmaceuticals CMO & CRO market: Service type outlook key takeaways
FIG. 22 U.S. biopharmaceuticals CMO & CRO market: Service movement analysis
FIG. 23 U.S. contract manufacturing market, 2018 - 2030 (USD Million)
FIG. 24 U.S. process development market, 2018 - 2030 (USD Million)
FIG. 25 U.S. downstream processing market, 2018 - 2030 (USD Million)
FIG. 26 U.S. upstream processing market, 2018 - 2030 (USD Million)
FIG. 27 U.S. fill & finish operations market, 2018 - 2030 (USD Million)
FIG. 28 U.S. analytical & QC testing market, 2018 - 2030 (USD Million)
FIG. 29 U.S. contract packaging market, 2018 - 2030 (USD Million)
FIG. 30 U.S. contract research market, 2018 - 2030 (USD Million)
FIG. 31 U.S. oncology market, 2018 - 2030 (USD Million)
FIG. 32 U.S. inflammation & immunology market, 2018 - 2030 (USD Million)
FIG. 33 U.S. cardiology market, 2018 - 2030 (USD Million)
FIG. 34 U.S. neuroscience market, 2018 - 2030 (USD Million)
FIG. 35 U.S. other CRO services market, 2018 - 2030 (USD Million)
FIG. 36 U.S. biopharmaceuticals CMO & CRO market: Product outlook key takeaways
FIG. 37 U.S. biopharmaceuticals CMO & CRO market: Product movement analysis
FIG. 38 U.S. biologics market, 2018 - 2030 (USD Million)
FIG. 39 Year-wise annual approvals for MAbs products that are currently marketed in U.S./EU
FIG. 40 Share of MAbs-related R&D programs at different pipeline phases, 2015
FIG. 41 U.S. monoclonal antibodies market, 2018 - 2030 (USD Million)
FIG. 42 Comparison of R&D biotech pipeline expansion for recombinant products
FIG. 43 U.S. recombinant proteins market, 2018 - 2030 (USD Million)
FIG. 44 U.S. vaccines market, 2018 - 2030 (USD Million)
FIG. 45 U.S. antisense, RNAi, &molecular therapy market, 2018 - 2030 (USD Million)
FIG. 46 U.S. other product market, 2018 - 2030 (USD Million)
FIG. 47 U.S. biosimilars market, 2018 - 2030 (USD Million)
Companies Mentioned
- Boehringer Ingelheim GmbH
- LONZA
- Catalent
- Rentschler Biotechnologie GmbH
- Thermo Fisher Scientific Inc
- AGC Biologics
- Fujifilm Diosynth Biotechnologies
- Abzena
- Samsung Biologics
- WuXi Biologics
- AbbVie Inc.
- Charles River Laboratories International, Inc.
- ICON Plc
- Parexel International Corporation
- Labcorp.
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 122 |
| Published | February 2023 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value ( USD | $ 10.1 Billion |
| Forecasted Market Value ( USD | $ 16.7 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | United States |
| No. of Companies Mentioned | 15 |