1. PREFACE
1.1. Chapter Overview
1.2. Key Insights
1.3. Scope of the Report
1.4. Research Methodology
1.5. Frequently Asked Questions
1.6. Chapter Outlines
3. INTRODUCTION
3.1. Chapter Overview
3.2. Introduction to Transfection
3.3. Methods of Transfection
3.3.1. Viral Transfection Systems
3.3.1.1. Types of Viral Transfection Vectors
3.3.1.1.1. Adeno-associated Virus-based Vectors
3.3.1.1.2. Adenovirus-based Vectors
3.3.1.1.3. Herpes Virus-based Vectors
3.3.1.1.4. Lentivirus-based Vectors
3.3.1.1.5. Retroviral-based Vectors
3.3.2. Non-Viral Transfection Systems
3.3.2.1. Chemical-based Transfection
3.3.2.1.1. Lipoplexes-based Transfection
3.3.2.1.2. Polyplexes-based Transfection
3.3.2.1.3. Lipo-polyplexes-based Transfection
3.3.2.1.4. Dendrimer-based Transfection
3.3.2.1.5. Cell Penetrating Peptide-based Transfection
3.3.2.2. Physical Transfection Systems
3.3.2.2.1. Electroporation-based Transfection
3.3.2.2.2. Gene Gun-based Transfection
3.3.2.2.3. Sonoporation-based Transfection
3.3.2.2.4. Microinjection-based Transfection
3.3.2.2.5. Magnetofection-based Transfection
3.4. Applications of Transfection
3.4.1. Advanced Therapeutic Medicinal Product Development
3.4.2. Gene Silencing
3.4.3. Generation of Stable Cell Lines
3.4.4. Large-scale Protein Production
3.4.5. Stem Cell Engineering
3.5. Future Perspectives
4. NON-VIRAL TRANSFECTION REAGENTS: MARKET LANDSCAPE
4.1. Chapter Overview
4.2. List of Non-Viral Transfection Reagents
4.2.1. Analysis by Type of Carrier Used
4.2.2. Analysis by Compatible Cell Type
4.2.3. Analysis by Type of Molecule Delivered
4.2.4. Analysis by Serum Compatibility
4.3 List of Non-Viral Transfection Reagent Developers
4.3.1. Analysis by Year of Establishment
4.3.2. Analysis by Company Size
4.3.3. Analysis by Location of Headquarters (Region-wise)
4.3.4. Analysis by Location of Headquarters (Country-wise)
5. ELECTROPORATION-BASED TRANSFECTION SYSTEMS: MARKET LANDSCAPE
5.1. Chapter Overview
5.2. List of Electroporation-based Transfection Systems
5.2.1. Analysis by Compatible Cell Type
5.2.2. Analysis by Type of Molecule Delivered
5.3. List of Electroporation-based Transfection System Developers
5.3.1. Analysis by Year of Establishment
5.3.2. Analysis by Company Size
5.2.3. Analysis by Location of Headquarters (Region -wise)
5.3.4. Analysis by Location of Headquarters (Country-wise)
6. OTHER NON-VIRAL TRANSFECTION SYSTEMS: MARKET LANDSCAPE
6.1. Chapter Overview
6.2. List of Other Non-Viral Transfection Systems
6.2.1. Analysis by Compatible Cell Type
6.2.2. Analysis by Type of Molecule Delivered
6.3. List of Other Non-Viral Transfection System Developers
6.3.1. Analysis by Year of Establishment
6.3.2. Analysis by Company Size
6.3.3. Analysis by Location of Headquarters (Region-wise)
6.3.4. Analysis by Location of Headquarters (Country-wise)
7. COMPANY COMPETITIVENESS ANALYSIS
7.1. Chapter Overview
7.2. Methodology and Key Parameters
7.3. Non-Viral Transfection Reagent Developers: Company Competitiveness Analysis
7.3.1. Non-Viral Transfection Reagent Developers based in North America
7.3.2. Non-Viral Transfection Reagent Developers based in Europe
7.3.3. Non-Viral Transfection Reagent Developers based in Asia-Pacific and Rest of the World
8. TECHNOLOGY COMPETITIVENESS ANALYSIS
8.1. Chapter Overview
8.2. Methodology and Key Parameters
8.3. Electroporation-based Transfection Systems: Technology Competitiveness Analysis
8.3.1. Technologies Offered by Players based in North America
8.3.2. Technologies Offered by Players based in Europe
8.3.3. Technologies Offered by Players based in Asia-Pacific and Rest of the World
8.4. Other Non-Viral Transfection Systems: Technology Competitiveness Analysis
8.4.1. Technologies Offered by Players based in North America
8.4.2. Technologies Offered by Players based in Europe
8.4.3. Technologies Offered by Players based in Asia-Pacific and Rest of the World
9. COMPANY PROFILES
9.1. Chapter Overview
9.2. Non-Viral Transfection Reagent Developers
9.2.1. MilliporeSigma
9.2.1.1. Company Overview
9.2.1.2. Financial Information
9.2.1.3. Recent Developments and Future Outlook
9.2.2. OZ Biosciences
9.2.2.1. Company Overview
9.2.2.2. Recent Developments and Future Outlook
9.2.3. Thermo Fisher Scientific
9.2.3.1. Company Overview
9.2.3.2. Financial Information
9.2.3.3. Recent Development and Future Outlook
9.3. Electroporation-based Transfection System Developers
9.3.1. BEX
9.3.1.1. Company Overview
9.3.1.2. Recent Developments and Future Outlook
9.3.2. Bio-Rad Laboratories
9.3.2.1. Company Overview
9.3.2.2. Financial Information
9.3.2.3. Recent Developments and Future Outlook
9.3.3. BTX (A subsidiary of Harvard Bioscience)
9.3.3.1. Company Overview
9.3.3.2. Recent Developments and Future Outlook
9.3.4. MaxCyte
9.3.4.1. Company Overview
9.3.4.2. Financial Information
9.3.4.3. Recent Developments and Future Outlook
9.3.5. NepaGene
9.3.5.1. Company Overview
6.3.5.2. Recent Developments and Future Outlook
9.4. Other Non-Viral Transfection System Developers
9.4.1. Imunon (Formerly known as Celsion)
9.4.1.1. Company Overview
9.4.1.2. Recent Developments and Future Outlook
9.4.2. Genprex
9.4.2.1. Company Overview
9.4.2.2. Financial Information
9.4.2.3. Recent Developments and Future Outlook
9.4.3. Inovio Pharmaceuticals
9.4.3.1. Company Overview
9.4.3.2. Financial Information
9.4.3.3. Recent Developments and Future Outlook
10. POTENTIAL STRATEGIC PARTNERS
10.1. Chapter Overview
10.2. Scope and Methodology
10.3. Non-Viral Transfection System Developers: Potential Strategic Partners in North America
10.3.1. Most Likely Partners
10.3.2. Likely Partners
10.3.3. Less Likely Partners
10.3.4. Least Likely Partners
10.4. Non-Viral Transfection System Developers: Potential Strategic Partners in Europe
10.4.1. Most Likely Partners
10.4.2. Likely Partners
10.4.3. Less Likely Partners
10.5. Non-Viral Transfection System Developers: Potential Strategic Partners in Asia-Pacific and Rest of the World
10.5.1. Most Likely Partners
10.5.2. Likely Partners
10.5.3. Less Likely Partners
11. BIG PHARMA INITIATIVES
11.1. Chapter Overview
11.2. Scope and Methodology
11.3. None-Viral Transfection Reagents and System Developers: Big Pharma Initiatives
11.3.1. Analysis by Year of Initiative
11.3.2. Analysis by Number of Initiative
11.3.3. Analysis by Type of Initiative
11.3.4. Analysis by Type of Therapy
11.3.5. Analysis by Target Therapeutic Area
12. PATENT ANALYSIS
12.1. Chapter Overview
12.2. Scope and Methodology
12.3. Non-Viral Transfection Reagents and Systems: Patent Analysis
12.3.1. Analysis by Publication Year
12.3.2. Analysis by Application Year
12.3.3. Analysis by Patent Jurisdiction
12.3.4. Analysis by Type of Applicant
12.3.5. Analysis by CPC Sections
12.3.6. Analysis by Emerging Focus Areas (Word Cloud Representation)
12.3.7. Leading Players: Analysis by Number of Patents
12.4. Non-Viral Transfection Reagents and Systems: Patent Benchmarking Analysis
12.4.1. Analysis by Patent Characteristics (CPC Symbols)
12.4.2. Analysis by Geography
12.5. Non-Viral Transfection Reagents and Systems: Patent Valuation Analysis
13. PUBLICATION ANALYSIS
13.1. Chapter Overview
13.2. Scope and Methodology
13.3. Non-Viral Transfection Reagents and Systems: Recent Publications
13.4. Analysis by Year of Publication
13.5. Analysis by Type of Publication
13.6. Analysis by Type of Molecule Delivered
13.7. Analysis by Target Therapeutic Area
13.8. Analysis by Key Focus Areas (Word Cloud Representation)
13.9. Analysis by Prominent Cells and Cell Lines (Word Cloud Representation)
13.1 Leading Publishers: Analysis by Number of Publications
13.11. Prominent Journals: Analysis by Number of Publications
13.12. Prominent Copyright Holders: Analysis by Number of Publications
13.13 Key Funding Institutes: Analysis by Number of Publications
14. PRICING STRATEGY FRAMEWORK
14.1. Chapter Overview
14.2 Roots Analysis Framework
14.2.1. Methodology
14.2.2. Theoretical Framework and Price Evaluation Hypothesis
14.2.3. Results and Interpretation
14.2.3.1. Product Price Evaluation Matrix: Based on Transfection Efficiency
14.2.3.2. Product Price Evaluation Matrix: Based on Compatible Cell Type
14.2.3.3. Product Price Evaluation Matrix: Based on Type of Carrier Used
14.2.3.4. Product Price Evaluation Matrix: Based on Type of Molecule Delivered
14.2.3.5. Product Price Evaluation Matrix: Based on Serum Compatibility
14.3. Concluding Remarks
15. MARKET SIZING AND OPPORTUNITY ANALYSIS
15.1. Chapter Overview
15.2. Forecast Methodology and Key Assumptions
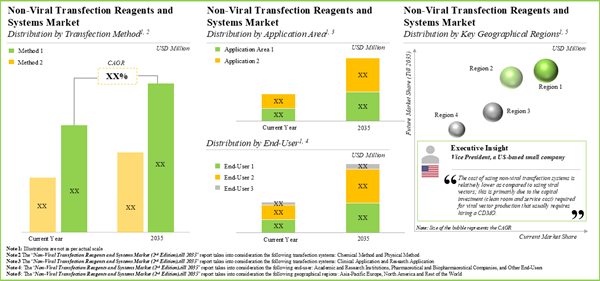
15.3. Non-Viral Transfection Reagents and Systems Market, 2023-2035
15.4. Non-Viral Transfection Reagents and Systems Market: Analysis by Type of Non-Viral Transfection Method, 2023 and 2035
15.5. Non-Viral Transfection Reagents and Systems Market: Analysis by End-User, 2023 and 2035
15.6. Non-Viral Transfection Reagents and Systems Market: Analysis by Application Area, 2023 and 2035
15.7. Non-Viral Transfection Reagents and Systems Market: Analysis by Key Geographical Regions, 2023 and 2035
15.7.1. Non-Viral Transfection Reagents and Systems Market in North America, 2023-2035
15.7.1.1. Non-Viral Transfection Reagents and Systems Market in North America: Analysis by Type of End-User, 2023-2035
15.7.1.1.1. Non-Viral Transfection Reagents and Systems Market in North America for Pharmaceutical Companies, 2023-2035
15.7.1.1.2. Non-Viral Transfection Reagents and Systems Market in North America for Academic and Research Institutions, 2023-2035
15.7.1.1.3. Non-Viral Transfection Reagents and Systems Market in North America for Other End-Users, 2023-2035
15.7.1.2. Non-Viral Transfection Reagents and Systems Market in North America: Analysis by Application Area, 2023-2035
15.7.1.2.1. Non-Viral Transfection Reagents and Systems Market in North America for Research Applications, 2023-2035
15.7.1.2.2. Non-Viral Transfection Reagents and Systems Market in North America for Clinical Applications, 2023-2035
15.7.2. Non-Viral Transfection Reagents and Systems Market in Europe, 2023-2035
15.7.2.1. Non-Viral Transfection Reagents and Systems Market in Europe: Analysis by Type of End-User, 2023-2035
15.7.2.1.1. Non-Viral Transfection Reagents and Systems Market in Europe for Pharmaceutical Companies, 2023-2035
15.7.2.1.2. Non-Viral Transfection Reagents and Systems Market in Europe for Academic and Research Institutions, 2023-2035
15.7.2.1.3. Non-Viral Transfection Reagents and Systems Market in Europe for Other End-Users, 2023-2035
15.7.2.2. Non-Viral Transfection Reagents and Systems Market in Europe: Analysis by Application Area, 2023-2035
15.7.2.2.1. Non-Viral Transfection Reagents and Systems Market in Europe for Research Applications, 2023-2035
15.7.2.2.2. Non-Viral Transfection Reagents and Systems Market in Europe for Clinical Applications, 2023-2035
15.7.3. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific, 2023-2035
15.7.3.1. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific: Analysis by Type of End-User, 2023-2035
15.7.3.1.1. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Pharmaceutical Companies, 2023-2035
15.7.3.1.2. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Academic and Research Institutions, 2023-2035
15.7.3.1.3. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Other End-Users, 2023-2035
15.7.3.2. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific: Analysis by Application Area, 2023-2035
15.7.3.2.1. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Research Applications, 2023-2035
15.7.3.2.2. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Clinical Applications, 2023-2035
15.7.4. Non-Viral Transfection Reagents and Systems Market in Rest of the World, 2023-2035
15.7.4.1. Non-Viral Transfection Reagents and Systems Market in Rest of the World: Analysis by Type of End-User, 2023-2035
15.7.4.1.1. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Pharmaceutical Companies, 2023-2035
15.7.4.1.2. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Academic and Research Institutions, 2023-2035
15.7.4.1.3. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Other End-Users, 2023-2035
15.7.4.2. Non-Viral Transfection Reagents and Systems Market in Rest of the World: Analysis by Application Area, 2023-2035
15.7.4.2.1. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Research Applications, 2023-2035
15.7.4.2.2. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Clinical Applications, 2023-2035
LIST OF FIGURES
Figure 2.1 Executive Summary: Current Landscape of Non-Viral Transfection Reagents
Figure 2.2 Executive Summary: Current Landscape of Electroporation-based Non-Viral Transfection Systems
Figure 2.3 Executive Summary: Current Landscape of Other Non-Viral Transfection Systems
Figure 2.4 Executive Summary: Big Pharma Initiatives
Figure 2.5 Executive Summary: Patent Analysis
Figure 2.6 Executive Summary: Publication Analysis
Figure 2.7 Executive Summary: Market Forecast and Opportunity Analysis
Figure 3.1 Commonly Used Viral Vectors
Figure 3.2 Types of Non-Viral Transfection Methods
Figure 3.3 Electroporation Mechanism
Figure 3.4 Gene Gun Mechanism
Figure 3.5 Sonoporation Mechanism
Figure 3.6 Microinjection Mechanism
Figure 3.7 Magnetofection Mechanism
Figure 3.8 Applications of Transfection
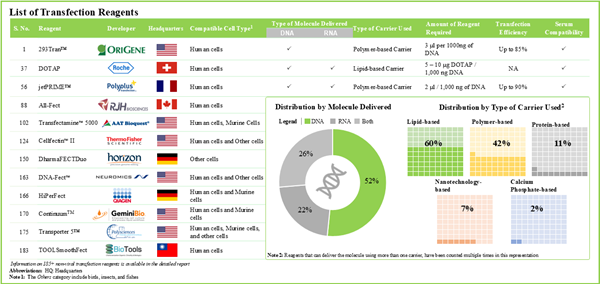
Figure 4.1 Non-Viral Transfection Reagents: Distribution by Type of Carrier Used
Figure 4.2 Non-Viral Transfection Reagents: Distribution by Compatible Cell Type
Figure 4.3 Non-Viral Transfection Reagents: Distribution by Type of Molecule Delivered
Figure 4.4 Non-Viral Transfection Reagents: Distribution by Serum Compatibility
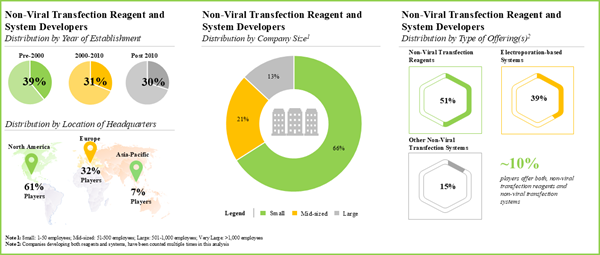
Figure 4.5 Non-Viral Transfection Reagent Developers: Distribution by Year of Establishment
Figure 4.6 Non-Viral Transfection Reagent Developers: Distribution by Company Size
Figure 4.7 Non-Viral Transfection Reagent Developers: Distribution by Location of Headquarters (Region-wise)
Figure 4.8 Non-Viral Transfection Reagent Developers: Distribution by Location of Headquarters (Country-wise)
Figure 5.1 Electroporation-based Transfection Systems: Distribution by Compatible Cell Type
Figure 5.2 Electroporation-based Transfection Systems: Distribution by Type of Molecule Delivered
Figure 5.3 Electroporation-based Transfection System Developers: Distribution by Year of Establishment
Figure 5.4 Electroporation-based Transfection System Developers: Distribution by Company Size
Figure 5.5 Electroporation-based Transfection System Developers: Distribution by Location of Headquarters (Region-wise)
Figure 5.6 Electroporation-based Transfection System Developers: Distribution by Location of Headquarters (Country-wise)
Figure 6.1 Other Non-Viral Transfection Systems: Distribution by Compatible Cell Type
Figure 6.2 Other Non-Viral Transfection Systems: Distribution by Type of Molecule Delivered
Figure 6.3 Other Non-Viral Transfection Systems: Distribution by Year of Establishment
Figure 6.4 Other Non-Viral Transfection System Developers: Distribution by Company Size
Figure 6.5 Other Non-Viral Transfection System Developers: Distribution by Location of Headquarters (Region-wise)
Figure 6.6 Other Non-Viral Transfection System Developers: Distribution by Location of Headquarters (Country-wise)
Figure 7.1 Company Competitiveness Analysis: Distribution of Non-Viral Transfection Reagent Developers based in North America
Figure 7.2 Company Competitiveness Analysis: Distribution of Non-Viral Transfection Reagent Developers based in Europe
Figure 7.3 Company Competitiveness Analysis: Distribution of Non-Viral Transfection Reagent Developers based in Asia-Pacific and Rest of the World
Figure 8.1 Electroporation-based Systems: Distribution of Technologies Offered by Players based in North America
Figure 8.2 Electroporation-based Systems: Distribution of Technologies Offered by Players based in Europe
Figure 8.3 Electroporation-based Systems: Distribution of Technologies Offered by Players based in Asia-Pacific and Rest of the World
Figure 8.4 Other Non-Viral Transfection Systems: Distribution of Technologies Offered by Players based in North America
Figure 8.5 Other Non-Viral Transfection Systems: Distribution of Technologies Offered by Players based in Europe
Figure 8.6 Other Non-Viral Transfection Systems: Distribution of Technologies Offered by Players based in Asia-Pacific and Rest of the World
Figure 9.1 MilliporeSigma: Annual Revenues, 2016-H1 2022 (USD Billion)
Figure 9.2 Thermo Fisher Scientific: Annual Revenues, 2016- H1 2022 (USD Billion)
Figure 9.3 Bio-Rad Laboratories: Annual Revenues, 2016- H1 2022 (USD Million)
Figure 9.4 MaxCyte: Annual Revenues, 2016- H1 2022 (USD Million)
Figure 9.5 Genprex: Annual Revenues, 2016- H1 2022 (USD Million)
Figure 9.6 Inovio Pharmaceuticals: Annual Revenues, 2016- H1 2022 (USD Million)
Figure 11.1 Big Pharma Initiatives: Cumulative Distribution by Year of Initiative
Figure 11.2 Big Pharma Initiatives: Distribution by Number of Initiatives
Figure 11.3 Big Pharma Initiatives: Distribution by Type of Initiative
Figure 11.4 Big Pharma Initiatives: Distribution by Type of Therapy
Figure 11.5 Big Pharma Initiatives: Distribution by Target Therapeutic Area
Figure 12.1 Patent Analysis: Distribution by Type of Patent
Figure 12.2 Patent Analysis: Distribution by Publication Year
Figure 12.3 Patent Analysis: Distribution by Application Year
Figure 12.4 Patent Analysis: Distribution by Patent Jurisdiction
Figure 12.5 Patent Analysis: Distribution by Type of Applicant
Figure 12.6 Patent Analysis: Distribution by CPC Sections
Figure 12.7 Word Cloud: Emerging Focus Areas
Figure 12.8 Leading Players (Industry): Distribution by Number of Patents
Figure 12.9 Leading Players (Non-Industry): Distribution by Number of Patents
Figure 12.10 Patent Benchmarking: Distribution by Patent Characteristics
Figure 12.11 Patent Analysis (Leading Industry Players): Benchmarking by CPC Symbols
Figure 12.12 Patent Analysis: Distribution by Patent Age
Figure 12.13 Non-Viral Transfection Reagents and Systems: Patent Valuation
Figure 13.1 Publication Analysis: Cumulative Year-wise Trend
Figure 13.2 Publication Analysis: Distribution by Type of Publication
Figure 13.3 Publication Analysis: Distribution by Type of Molecule Delivered
Figure 13.4 Publication Analysis: Distribution by Target Therapeutic Area
Figure 13.5 Word Cloud: Key Focus Areas
Figure 13.6 Word Cloud: Popular Cells and Cell Lines
Figure 13.7 Leading Publishers: Distribution by Number of Publications
Figure 13.8 Prominent Journals: Distribution by Number of Publications
Figure 13.9 Prominent Copyright Holders: Distribution by Number of Publications
Figure 13.10 Key Funding Institutes: Distribution by Number of Publications
Figure 14.1 Non-Viral Transfection Reagents and Systems: Roots Analysis Pricing Strategy Framework
Figure 14.2 Non-Viral Transfection Reagents and Systems: Roots Analysis Pricing Strategy Graphical Interpretation
Figure 15.1 Overall Non-Viral Transfection Reagents and Systems Market, 2023-2035 (USD Million)
Figure 15.2 Non-Viral Transfection Systems Market: Distribution by Type of Non-Viral Transfection Method, 2023 and 2035 (USD Million)
Figure 15.3 Non-Viral Transfection Systems Market: Distribution by End-User, 2023 and 2035 (USD Million)
Figure 15.4 Non-Viral Transfection Systems Market: Distribution by Application Area, 2023 and 2035 (USD Million)
Figure 15.5 Non-Viral Transfection Systems Market: Distribution by Key Geographical Regions, 2023 and 2035 (USD Million)
Figure 15.6 Non-Viral Transfection Systems Market in North America, 2023-2035 (USD Million)
Figure 15.7 Non-Viral Transfection Systems Market in North America for Pharmaceutical Companies, 2023-2035 (USD Million)
Figure 15.8 Non-Viral Transfection Systems Market in North America for Academic and Research Institutions, 2023-2035 (USD Million)
Figure 15.9 Non-Viral Transfection Systems Market in North America for Other End-Users, 2023-2035 (USD Million)
Figure 15.10 Non-Viral Transfection Systems Market in North America for Research Applications, 2023-2035 (USD Million)
Figure 15.11 Non-Viral Transfection Systems Market in North America for Clinical Applications, 2023-2035 (USD Million)
Figure 15.12 Non-Viral Transfection Systems Market in Europe, 2023-2035 (USD Million)
Figure 15.13 Non-Viral Transfection Systems Market in Europe for Pharmaceutical Companies, 2023-2035 (USD Million)
Figure 15.14 Non-Viral Transfection Systems Market in Europe for Academic and Research Institutions, 2023-2035 (USD Million)
Figure 15.15 Non-Viral Transfection Systems Market in Europe for Other End-Users, 2023-2035 (USD Million)
Figure 15.16 Non-Viral Transfection Systems Market in Europe for Research Applications, 2023-2035 (USD Million)
Figure 15.17 Non-Viral Transfection Systems Market in Europe for Clinical Applications, 2023-2035 (USD Million)
Figure 15.18 Non-Viral Transfection Systems Market in Asia-Pacific, 2023-2035 (USD Million)
Figure 15.19 Non-Viral Transfection Systems Market in Asia-Pacific for Pharmaceutical Companies, 2023-2035 (USD Million)
Figure 15.20 Non-Viral Transfection Systems Market in Asia-Pacific for Academic and Research Institutions, 2023-2035 (USD Million)
Figure 15.21 Non-Viral Transfection Systems Market in Asia-Pacific for Other End-Users, 2023-2035 (USD Million)
Figure 15.22 Non-Viral Transfection Systems Market in Asia-Pacific for Research Applications, 2023-2035 (USD Million)
Figure 15.23 Non-Viral Transfection Systems Market in Asia-Pacific for Clinical Applications, 2023-2035 (USD Million)
Figure 15.24 Non-Viral Transfection Systems Market in Rest of the World, 2023-2035 (USD Million)
Figure 15.25 Non-Viral Transfection Systems Market in Rest of the World for Pharmaceutical Companies, 2023-2035 (USD Million)
Figure 15.26 Non-Viral Transfection Systems Market in Rest of the World for Academic and Research Institutions, 2023-2035 (USD Million)
Figure 15.27 Non-Viral Transfection Systems Market in Rest of the World for Other End-Users, 2023-2035 (USD Million)
Figure 15.28 Non-Viral Transfection Systems Market in Rest of the World for Research Applications, 2023-2035 (USD Million)
Figure 15.29 Non-Viral Transfection Systems Market in Rest of the World for Clinical Applications, 2023-2035 (USD Million)
Figure 17.1. Conclusion: Current Landscape of Non-Viral Transfection Reagents
Figure 17.2 Conclusion: Current Landscape of Electroporation-based Non-Viral Transfection Systems
Figure 17.3 Conclusion: Current Landscape of Other Non-Viral Transfection Systems
Figure 17.4 Conclusion: Big Pharma Initiatives
Figure 17.5 Conclusion: Patent Analysis
Figure 17.6 Conclusion: Publication Analysis
Figure 17.7 Conclusion: Market Forecast and Opportunity Analysis
LIST OF TABLES
Table 3.1. Specifications of Viral Vectors
Table 4.1. Non-Viral Transfection Reagents: Information on Type of Carrier Used, Compatible Cell Type and Volume of Reagent (per unit)
Table 4.2. Non-Viral Transfection Reagents: Information on Type of Molecule Delivered, Serum Compatibility and Transfection Efficiency
Table 4.3. Non-Viral Transfection Reagents: Information on Storage Temperature, Number of Transfections Per Unit Volume of Reagent and Cost of Reagent (USD)
Table 4.4. Non-Viral Transfection Reagent Developers: Information on Year of Establishment, Company Size and Location of Headquarters
Table 5.1. Electroporation-based Transfection Systems: Information on Compatible Cell Type and Type of Molecule Delivered
Table 5.2. Electroporation-based Transfection Systems: Information Output Voltage, Pulse and Cost of System (USD)
Table 5.3. Electroporation-based Transfection System Developers: Information on Year of Establishment, Company Size and Location of Headquarters
Table 6.1. Other Non-Viral Transfection Systems: Information on Compatible Cell Type and Type of Molecule Delivered
Table 6.2 Other Non-Viral Transfection System Developers: Information on Year of Establishment, Company Size and Location of Headquarters
Table 9.1. List of Companies Profiled
Table 9.2. Altogen Biosystems: Company Snapshot
Table 9.3. Altogen Biosystems: Recent Developments and Future Outlook
Table 9.4. Millipore Sigma: Company Snapshot
Table 9.5. Millipore Sigma: Recent Developments and Future Outlook
Table 9.6. OZ Biosciences: Company Snapshot
Table 9.7. OZ Biosciences: Recent Developments and Future Outlook
Table 9.8. Thermo Fisher Scientific: Company Snapshot
Table 9.9. Thermo Fisher Scientific: Recent Developments and Future Outlook
Table 9.10. BEX: Company Snapshot
Table 9.11. BEX: Recent Developments and Future Outlook
Table 9.12. Bio-Rad Laboratories: Company Snapshot
Table 9.13. Bio-Rad Laboratories: Recent Developments and Future Outlook
Table 9.14. BTX: Company Snapshot
Table 9.15. BTX: Recent Developments and Future Outlook
Table 9.16. MaxCyte: Company Snapshot
Table 9.17. MaxCyte: Recent Developments and Future Outlook
Table 9.18. NepaGene: Company Snapshot
Table 9.19. NepaGene: Recent Developments and Future Outlook
Table 9.20. Imunon: Company Snapshot
Table 9.21. Imunon: Recent Developments and Future Outlook
Table 9.22. Genprex: Company Snapshot
Table 9.23. Genprex: Recent Developments and Future Outlook
Table 9.24. Inovio Pharmaceuticals: Company Snapshot
Table 9.25. Inovio Pharmaceuticals: Recent Developments and Future Outlook
Table 10.1. Most Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in North America
Table 10.2. Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in North America
Table 10.3. Less Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in North America
Table 10.4. Least Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in North America
Table 10.5. Most Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in Europe
Table 10.6. Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in Europe
Table 10.7. Less Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in Europe
Table 10.8. Most Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in Asia-Pacific and Rest of the World
Table 10.9. Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in Asia-Pacific and Rest of the World
Table 10.10. Less Likely Partners: Non-Viral Vector-based Therapy Developers Headquartered in Asia-Pacific and Rest of the World
Table 11.1. Big Pharma Initiatives: List of Non-Viral Transfection Focused Initiatives
Table 12.1. Patent Analysis: Top CPC Sections
Table 12.2. Patent Analysis: Top CPC Symbols
Table 12.3. Patent Analysis: Top CPC Codes
Table 12.4. Patent Analysis: Summary of Benchmarking Analysis
Table 12.5. Patent Analysis: List of Leading Patents (by Highest Relative Valuation)
Table 14.1. Product Price Evaluation Matrix: Based on Transfection Efficiency
Table 14.2. Product Price Evaluation Matrix: Based on Compatible Cell Type
Table 14.3. Product Price Evaluation Matrix: Based on Type of Carrier Used
Table 14.4. Product Price Evaluation Matrix: Based on Type of Molecule Delivered
Table 14.5. Product Price Evaluation Matrix: Based on Serum Compatibility
Table 18.1. Non-Viral Transfection Reagents: Distribution by Type of Carrier Used
Table 18.2. Non-Viral Transfection Reagents: Distribution by Compatible Cell Type
Table 18.3. Non-Viral Transfection Reagents: Distribution by Type of Molecule Delivered
Table 18.4. Non-Viral Transfection Reagents: Distribution by Serum Compatibility
Table 18.5. Non-Viral Transfection Reagent Developers: Distribution by Year of Establishment
Table 18.6. Non-Viral Transfection Reagent Developers: Distribution by Company Size
Table 18.7. Non-Viral Transfection Reagent Developers: Distribution by Location of Headquarters (Region-wise)
Table 18.8. Non-Viral Transfection Reagent Developers: Distribution by Location of Headquarters (Country-wise)
Table 18.9. Electroporation-based Transfection Systems: Distribution by Compatible Cell Type
Table 18.10. Electroporation-based Transfection Systems: Distribution by Type of Molecule Delivered
Table 18.11. Electroporation-based Transfection System Developers: Distribution by Year of Establishment
Table 18.12. Electroporation-based Transfection System Developers: Distribution by Company Size
Table 18.13. Electroporation-based Transfection System Developers: Distribution by Location of Headquarters (Region-wise)
Table 18.14. Electroporation-based Transfection System Developers: Distribution by Location of Headquarters (Country-wise)
Table 18.15. Other Non-Viral Transfection Systems: Distribution by Compatible Cell Type
Table 18.16. Other Non-Viral Transfection Systems: Distribution by Type of Molecule Delivered
Table 18.17. Other Non-Viral Transfection Systems: Distribution by Year of Establishment
Table 18.18. Other Non-Viral Transfection System Developers: Distribution by Company Size
Table 18.19. Other Non-Viral Transfection System Developers: Distribution by Location of Headquarters (Region-wise)
Table 18.20. Other Non-Viral Transfection System Developers: Distribution by Location of Headquarters (Country-wise)
Table 18.21 MilliporeSigma: Annual Revenues, 2016-H1 2022 (USD Billion)
Table 18.22 Thermo Fisher Scientific: Annual Revenues, 2016- H1 2022 (USD Billion)
Table 18.23 Bio-Rad Laboratories: Annual Revenues, 2016- H1 2022 (USD Million)
Table 18.24 MaxCyte: Annual Revenues, 2016- H1 2022 (USD Million)
Table 18.25 Genprex: Annual Revenues, 2016- H1 2022 (USD Million)
Table 18.26 Inovio Pharmaceuticals: Annual Revenues, 2016- H1 2022 (USD Million)
Table 18.27 Big Pharma Initiatives: Cumulative Distribution by Year of Initiative
Table 18.28 Big Pharma Initiatives: Distribution by Number of Initiatives
Table 18.29 Big Pharma Initiatives: Distribution by Type of Initiative
Table 18.30 Big Pharma Initiatives: Distribution by Type of Therapy
Table 18.31 Big Pharma Initiatives: Distribution by Target Therapeutic Area
Table 18.32 Patent Analysis: Distribution by Type of Patent
Table 18.33 Patent Analysis: Distribution by Publication Year
Table 18.34 Patent Analysis: Distribution by Application Year
Table 18.35 Patent Analysis: Distribution by Patent Jurisdiction
Table 18.36 Patent Analysis: Distribution by Type of Applicant
Table 18.37 Patent Analysis: Distribution by CPC Sections
Table 18.38 Leading Players (Industry): Distribution by Number of Patents
Table 18.39 Leading Players (Non-Industry): Distribution by Number of Patents
Table 18.40 Patent Benchmarking: Distribution by Patent Characteristics
Table 18.41 Patent Analysis (Leading Industry Players): Benchmarking by CPC Symbols
Table 18.42 Patent Analysis: Distribution by Patent Age
Table 18.43 Non-Viral Transfection Reagents and Systems: Patent Valuation
Table 18.44 Publication Analysis: Cumulative Year-wise Trend
Table 18.45 Publication Analysis: Distribution by Type of Publication
Table 18.46 Publication Analysis: Distribution by Type of Molecule Delivered
Table 18.47 Publication Analysis: Distribution by Target Therapeutic Area
Table 18.48 Leading Publishers: Distribution by Number of Publications
Table 18.49 Prominent Journals: Distribution by Number of Publications
Table 18.50 Prominent Copyright Holders: Distribution by Number of Publications
Table 18.51 Key Funding Institutes: Distribution by Number of Publications
Table 18.52 Overall Non-Viral Transfection Reagents and Systems Market, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.53 Non-Viral Transfection Systems Market, Conservative, Base and Optimistic Scenario: Distribution by Type of Non-Viral Transfection Method, 2023 and 2035 (USD Million)
Table 18.54 Non-Viral Transfection Systems Market, Conservative, Base and Optimistic Scenario: Distribution by End-User, 2023 and 2035 (USD Million)
Table 18.55 Non-Viral Transfection Systems Market, Conservative, Base and Optimistic Scenario: Distribution by Application Area, 2023 and 2035 (USD Million)
Table 18.56 Non-Viral Transfection Systems Market, Conservative, Base and Optimistic Scenario: Distribution by Key Geographical Regions, 2023 and 2035 (USD Million)
Table 18.57 Non-Viral Transfection Systems Market in North America, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.58 Non-Viral Transfection Systems Market in North America for Pharmaceutical Companies, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.59 Non-Viral Transfection Systems Market in North America for Academic and Research Institutions, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.60 Non-Viral Transfection Systems Market in North America for Other End-Users, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.61 Non-Viral Transfection Systems Market in North America for Research Applications, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.62 Non-Viral Transfection Systems Market in North America for Clinical Applications, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.63 Non-Viral Transfection Systems Market in Europe, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.64 Non-Viral Transfection Systems Market in Europe for Pharmaceutical Companies, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.65 Non-Viral Transfection Systems Market in Europe for Academic and Research Institutions, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.66 Non-Viral Transfection Systems Market in Europe for Other End-Users, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.67 Non-Viral Transfection Systems Market in Europe for Research Applications, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.68 Non-Viral Transfection Systems Market in Europe for Clinical Applications, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.69 Non-Viral Transfection Systems Market in Asia-Pacific, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.70 Non-Viral Transfection Systems Market in Asia-Pacific for Pharmaceutical Companies, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.71 Non-Viral Transfection Systems Market in Asia-Pacific for Academic and Research Institutions, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.72 Non-Viral Transfection Systems Market in Asia-Pacific for Other End-Users, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.73 Non-Viral Transfection Systems Market in Asia-Pacific for Research Applications, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.74 Non-Viral Transfection Systems Market in Asia-Pacific for Clinical Applications, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.75 Non-Viral Transfection Systems Market in Rest of the World, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.76 Non-Viral Transfection Systems Market in Rest of the World for Pharmaceutical Companies, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.77 Non-Viral Transfection Systems Market in Rest of the World for Academic and Research Institutions, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.78 Non-Viral Transfection Systems Market in Rest of the World for Other End-Users, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.79 Non-Viral Transfection Systems Market in Rest of the World for Research Applications, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)
Table 18.80 Non-Viral Transfection Systems Market in Rest of the World for Clinical Applications, Conservative, Base and Optimistic Scenario, 2023-2035 (USD Million)