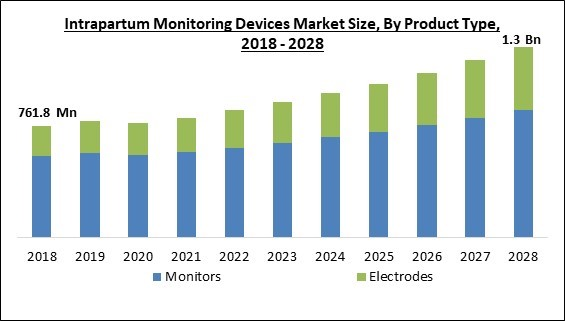
The Global Intrapartum Monitoring Devices Market size is expected to reach $1.3 billion by 2028, rising at a market growth of 7.0% CAGR during the forecast period.
The purpose of intrapartum monitoring devices is to track the temporal association between uterine contractions and myometrial activity. This is achieved by monitoring the fetal heart rate (FHR) changes with the help of Doppler ultrasound or direct fetal ECG measurement with a fetal scalp electrode. The correlation between the two tracks determines how the data should be interpreted. Additionally, finding newborns that may be hypoxic is an important deployment of intrapartum monitoring devices as it allows more evaluation of fetal health.
If not, a vaginal delivery or a cesarean section can be used to deliver the child. The external devices used for intrapartum monitoring involve attaching an ultrasound probe, also known as a transducer, to the female's belly. It transmits the infant's heartbeat sounds to a computer. The baby's heart rate is displayed on a screen along with its frequency. The vast pool of birth-related diseases, including premature births, promotes the usage of intrapartum monitoring devices.
In addition, growth and advancements in the medical device industry are also driving the use of intrapartum monitoring technologies. The use of intrapartum monitoring devices is predicted to expand due to the high potential for technology adoption in developing nations brought on by an increase in the practice of fetal health monitoring and an increase in the prevalence of birth disorders.
Additionally, the demand for improved medical technologies is growing, governments are making sizable investments to improve fetal health monitoring, and biotechnological industries are becoming more established in emerging economies. All of these factors contribute to the significant growth of the healthcare sector in emerging economies. Furthermore, it is believed that the cost-effectiveness of intrapartum monitoring devices will continue to promote market expansion. The increasing fetal monitoring device adoption among couples as a safety measure for their child and a rise in the knowledge of the hazards associated with birth diseases have raised the demand for monitoring systems.
COVID-19 Impact Analysis
The intrapartum monitoring devices market saw an adverse effect of the COVID-19 pandemic. This was due to COVID-19, which prevented individuals from traveling to clinics or hospitals for any less serious condition treatment process, including assessing the mother's and fetus's health. As a result, those who needed intrapartum monitoring services had to wait or put off the surgeries. Additionally, this situation was more observable in developing or underdeveloped nations as patients in industrialized regions had the option of at-home monitoring, which was unavailable in underdeveloped or emerging nations.
Market Growth Factors
High clinical benefits of intrapartum monitoring devices
Due to the need for better maternal healthcare and the rising number of issues from premature birth, the demand for intrapartum monitoring devices has increased. Healthcare spending is rising in developing countries, and patients are getting more knowledgeable about the advantages of adopting monitoring devices, giving market players more opportunities. The market is expanding as a result of rising risks and problems like brain injury, cerebral palsy, neonatal seizures, or fetal mortality during labor.
Increased use of infertility treatments and growing premature birth rates
Numerous studies have revealed several factors, including high blood pressure, prolonged pre-labor membrane rupturing, antepartum hemorrhage, multiple gestations, urinary tract infections (UTIs), short services, assisted reproductive technology, and placenta previa, are contributing to the rise in preterm births. In addition, modern medical technology has been included in fetal monitoring systems due to the rising number of preterm births. According to the WHO, approximately 15 million preterm births occur annually, and preterm births frequently result in neurological or physical abnormalities.
Market Restraining Factors
Exorbitant prices for monitoring equipment
The high cost of these devices and the rising worries about monitoring radiation having an impact on patients' health are proving to be prominent adverse factors for the growth of the market.The expansion of the market is further constrained by a shortage of qualified obstetric doctors in underdeveloped nations. Additionally, not many people in underdeveloped and developing nations are unaware of the availability of such devices. Moreover, more people give birth at home in these nations, slowing the demand for monitoring devices in hospitals and clinics.
Product Type Outlook
Based on product type, the intrapartum monitoring devices market is categorized into monitors and electrodes. The monitors segment garnered the highest revenue share in the intrapartum monitoring devices market in 2021. The growth of the segmentis because more women are using intrapartum monitors to check on the health of their unborn babies. Additionally, a variety of devices are available that have been created by different major market players. The need for at-home fetal health monitoring devices is further increased by the growing practice of doing so.
Method Outlook
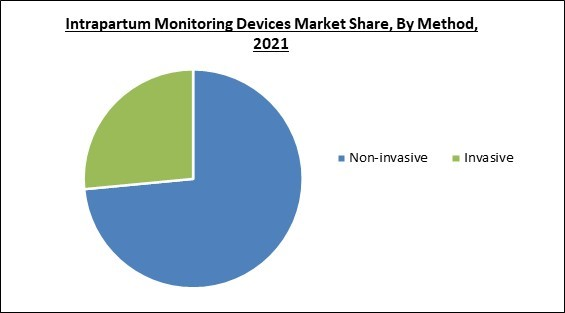
On the basis of method, the intrapartum monitoring devices market is divided into invasive and non-invasive. The invasive segment recorded a significant revenue share in the intrapartum monitoring devices market in 2021. The best registration quality is achieved by using invasive internal monitors. The growth of the segment is owed to the higher efficacy and reliability of invasive methods. Monitoring devices like fetal electrocardiograms (fECGs) are directly recorded from a fetus' scalp in the invasive mode. During an invasive fetal electrocardiogram (ECG), the membranes must be torn to introduce electrodes through the cervix and position them on the fetus's scalp.
End-user Outlook
Based on end user, the intrapartum monitoring devices market is segmented into hospitals, maternity centers, and others. The hospitals segment witnessed the maximum revenue share in the intrapartum monitoring devices market in 2021. Growth is attributable to the population's choice for intrapartum surveillance in hospitals and the ease with which patients can now visit hospitals and clinics following the COVID-19 outbreak. The clean and hygienic environment of the hospital allows for lesser chances of conceiving any infection. Additionally, in many developing nations, most intrapartum monitoring devices can only be accessed in hospital settings.
Regional Outlook
On the basis of region, the intrapartum monitoring devices market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North American segment procured the highest revenue share in the intrapartum monitoring devices market in 2021. The high prevalence of birth problems, including preterm births, the rise in market participants, and the explosion in the variety of technologies readily accessible in the region are all responsible for the segment's growth. In addition, the intrapartum monitoring devices market's expansion is further driven by rising public awareness and government endeavors to provide better solutions for maternal care.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Koninklijke Philips N.V., MindChild Medical, Inc., Arjo AB (Huntleigh Healthcare Ltd.), Stalwart Meditech Private Limited, MedGyn Products, Inc., Cardinal Health, Inc., GE HealthCare Technologies, Inc., CooperVision, Inc. (THE COOPER COMPANIES, INC.), and Olympus Corporation.
Scope of the Study
By Product Type
- Monitors
- Electrodes
By Method
- Non-invasive
- Invasive
By End-user
- Hospitals
- Maternity Centers
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Koninklijke Philips N.V.
- MindChild Medical, Inc.
- Arjo AB (Huntleigh Healthcare ltd.)
- Stalwart Meditech Private Limited
- MedGyn Products, Inc.
- Cardinal Health, Inc.
- GE HealthCare Technologies, Inc.
- CooperVision, Inc. (THE COOPER COMPANIES, INC.)
- Olympus Corporation
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology1.1 Market Definition
1.2 Objectives
1.3 Market Scope
1.4 Segmentation
1.4.1 Global Intrapartum Monitoring Devices Market, by Product Type
1.4.2 Global Intrapartum Monitoring Devices Market, by Method
1.4.3 Global Intrapartum Monitoring Devices Market, by End User
1.4.4 Global Intrapartum Monitoring Devices Market, by Geography
1.5 Methodology for the research
Chapter 2. Market Overview
2.1 Introduction
2.1.1 Overview
2.1.1.1 Market Composition & Scenario
2.2 Key Factors Impacting the Market
2.2.1 Market Drivers
2.2.2 Market Restraints
Chapter 3. Global Intrapartum Monitoring Devices Market by Product Type
3.1 Global Monitors Market by Region
3.2 Global Electrodes Market by Region
Chapter 4. Global Intrapartum Monitoring Devices Market by Method
4.1 Global Non-invasive Market by Region
4.2 Global Invasive Market by Region
Chapter 5. Global Intrapartum Monitoring Devices Market by End User
5.1 Global Hospitals Market by Region
5.2 Global Maternity Centers Market by Region
5.3 Global Others Market by Region
Chapter 6. Global Intrapartum Monitoring Devices Market by Region
6.1 North America Intrapartum Monitoring Devices Market
6.1.1 North America Intrapartum Monitoring Devices Market by Product Type
6.1.1.1 North America Monitors Market by Country
6.1.1.2 North America Electrodes Market by Country
6.1.2 North America Intrapartum Monitoring Devices Market by Method
6.1.2.1 North America Non-invasive Market by Country
6.1.2.2 North America Invasive Market by Country
6.1.3 North America Intrapartum Monitoring Devices Market by End User
6.1.3.1 North America Hospitals Market by Country
6.1.3.2 North America Maternity Centers Market by Country
6.1.3.3 North America Others Market by Country
6.1.4 North America Intrapartum Monitoring Devices Market by Country
6.1.4.1 US Intrapartum Monitoring Devices Market
6.1.4.1.1 US Intrapartum Monitoring Devices Market by Product Type
6.1.4.1.2 US Intrapartum Monitoring Devices Market by Method
6.1.4.1.3 US Intrapartum Monitoring Devices Market by End User
6.1.4.2 Canada Intrapartum Monitoring Devices Market
6.1.4.2.1 Canada Intrapartum Monitoring Devices Market by Product Type
6.1.4.2.2 Canada Intrapartum Monitoring Devices Market by Method
6.1.4.2.3 Canada Intrapartum Monitoring Devices Market by End User
6.1.4.3 Mexico Intrapartum Monitoring Devices Market
6.1.4.3.1 Mexico Intrapartum Monitoring Devices Market by Product Type
6.1.4.3.2 Mexico Intrapartum Monitoring Devices Market by Method
6.1.4.3.3 Mexico Intrapartum Monitoring Devices Market by End User
6.1.4.4 Rest of North America Intrapartum Monitoring Devices Market
6.1.4.4.1 Rest of North America Intrapartum Monitoring Devices Market by Product Type
6.1.4.4.2 Rest of North America Intrapartum Monitoring Devices Market by Method
6.1.4.4.3 Rest of North America Intrapartum Monitoring Devices Market by End User
6.2 Europe Intrapartum Monitoring Devices Market
6.2.1 Europe Intrapartum Monitoring Devices Market by Product Type
6.2.1.1 Europe Monitors Market by Country
6.2.1.2 Europe Electrodes Market by Country
6.2.2 Europe Intrapartum Monitoring Devices Market by Method
6.2.2.1 Europe Non-invasive Market by Country
6.2.2.2 Europe Invasive Market by Country
6.2.3 Europe Intrapartum Monitoring Devices Market by End User
6.2.3.1 Europe Hospitals Market by Country
6.2.3.2 Europe Maternity Centers Market by Country
6.2.3.3 Europe Others Market by Country
6.2.4 Europe Intrapartum Monitoring Devices Market by Country
6.2.4.1 Germany Intrapartum Monitoring Devices Market
6.2.4.1.1 Germany Intrapartum Monitoring Devices Market by Product Type
6.2.4.1.2 Germany Intrapartum Monitoring Devices Market by Method
6.2.4.1.3 Germany Intrapartum Monitoring Devices Market by End User
6.2.4.2 UK Intrapartum Monitoring Devices Market
6.2.4.2.1 UK Intrapartum Monitoring Devices Market by Product Type
6.2.4.2.2 UK Intrapartum Monitoring Devices Market by Method
6.2.4.2.3 UK Intrapartum Monitoring Devices Market by End User
6.2.4.3 France Intrapartum Monitoring Devices Market
6.2.4.3.1 France Intrapartum Monitoring Devices Market by Product Type
6.2.4.3.2 France Intrapartum Monitoring Devices Market by Method
6.2.4.3.3 France Intrapartum Monitoring Devices Market by End User
6.2.4.4 Russia Intrapartum Monitoring Devices Market
6.2.4.4.1 Russia Intrapartum Monitoring Devices Market by Product Type
6.2.4.4.2 Russia Intrapartum Monitoring Devices Market by Method
6.2.4.4.3 Russia Intrapartum Monitoring Devices Market by End User
6.2.4.5 Spain Intrapartum Monitoring Devices Market
6.2.4.5.1 Spain Intrapartum Monitoring Devices Market by Product Type
6.2.4.5.2 Spain Intrapartum Monitoring Devices Market by Method
6.2.4.5.3 Spain Intrapartum Monitoring Devices Market by End User
6.2.4.6 Italy Intrapartum Monitoring Devices Market
6.2.4.6.1 Italy Intrapartum Monitoring Devices Market by Product Type
6.2.4.6.2 Italy Intrapartum Monitoring Devices Market by Method
6.2.4.6.3 Italy Intrapartum Monitoring Devices Market by End User
6.2.4.7 Rest of Europe Intrapartum Monitoring Devices Market
6.2.4.7.1 Rest of Europe Intrapartum Monitoring Devices Market by Product Type
6.2.4.7.2 Rest of Europe Intrapartum Monitoring Devices Market by Method
6.2.4.7.3 Rest of Europe Intrapartum Monitoring Devices Market by End User
6.3 Asia Pacific Intrapartum Monitoring Devices Market
6.3.1 Asia Pacific Intrapartum Monitoring Devices Market by Product Type
6.3.1.1 Asia Pacific Monitors Market by Country
6.3.1.2 Asia Pacific Electrodes Market by Country
6.3.2 Asia Pacific Intrapartum Monitoring Devices Market by Method
6.3.2.1 Asia Pacific Non-invasive Market by Country
6.3.2.2 Asia Pacific Invasive Market by Country
6.3.3 Asia Pacific Intrapartum Monitoring Devices Market by End User
6.3.3.1 Asia Pacific Hospitals Market by Country
6.3.3.2 Asia Pacific Maternity Centers Market by Country
6.3.3.3 Asia Pacific Others Market by Country
6.3.4 Asia Pacific Intrapartum Monitoring Devices Market by Country
6.3.4.1 China Intrapartum Monitoring Devices Market
6.3.4.1.1 China Intrapartum Monitoring Devices Market by Product Type
6.3.4.1.2 China Intrapartum Monitoring Devices Market by Method
6.3.4.1.3 China Intrapartum Monitoring Devices Market by End User
6.3.4.2 Japan Intrapartum Monitoring Devices Market
6.3.4.2.1 Japan Intrapartum Monitoring Devices Market by Product Type
6.3.4.2.2 Japan Intrapartum Monitoring Devices Market by Method
6.3.4.2.3 Japan Intrapartum Monitoring Devices Market by End User
6.3.4.3 India Intrapartum Monitoring Devices Market
6.3.4.3.1 India Intrapartum Monitoring Devices Market by Product Type
6.3.4.3.2 India Intrapartum Monitoring Devices Market by Method
6.3.4.3.3 India Intrapartum Monitoring Devices Market by End User
6.3.4.4 South Korea Intrapartum Monitoring Devices Market
6.3.4.4.1 South Korea Intrapartum Monitoring Devices Market by Product Type
6.3.4.4.2 South Korea Intrapartum Monitoring Devices Market by Method
6.3.4.4.3 South Korea Intrapartum Monitoring Devices Market by End User
6.3.4.5 Singapore Intrapartum Monitoring Devices Market
6.3.4.5.1 Singapore Intrapartum Monitoring Devices Market by Product Type
6.3.4.5.2 Singapore Intrapartum Monitoring Devices Market by Method
6.3.4.5.3 Singapore Intrapartum Monitoring Devices Market by End User
6.3.4.6 Malaysia Intrapartum Monitoring Devices Market
6.3.4.6.1 Malaysia Intrapartum Monitoring Devices Market by Product Type
6.3.4.6.2 Malaysia Intrapartum Monitoring Devices Market by Method
6.3.4.6.3 Malaysia Intrapartum Monitoring Devices Market by End User
6.3.4.7 Rest of Asia Pacific Intrapartum Monitoring Devices Market
6.3.4.7.1 Rest of Asia Pacific Intrapartum Monitoring Devices Market by Product Type
6.3.4.7.2 Rest of Asia Pacific Intrapartum Monitoring Devices Market by Method
6.3.4.7.3 Rest of Asia Pacific Intrapartum Monitoring Devices Market by End User
6.4 LAMEA Intrapartum Monitoring Devices Market
6.4.1 LAMEA Intrapartum Monitoring Devices Market by Product Type
6.4.1.1 LAMEA Monitors Market by Country
6.4.1.2 LAMEA Electrodes Market by Country
6.4.2 LAMEA Intrapartum Monitoring Devices Market by Method
6.4.2.1 LAMEA Non-invasive Market by Country
6.4.2.2 LAMEA Invasive Market by Country
6.4.3 LAMEA Intrapartum Monitoring Devices Market by End User
6.4.3.1 LAMEA Hospitals Market by Country
6.4.3.2 LAMEA Maternity Centers Market by Country
6.4.3.3 LAMEA Others Market by Country
6.4.4 LAMEA Intrapartum Monitoring Devices Market by Country
6.4.4.1 Brazil Intrapartum Monitoring Devices Market
6.4.4.1.1 Brazil Intrapartum Monitoring Devices Market by Product Type
6.4.4.1.2 Brazil Intrapartum Monitoring Devices Market by Method
6.4.4.1.3 Brazil Intrapartum Monitoring Devices Market by End User
6.4.4.2 Argentina Intrapartum Monitoring Devices Market
6.4.4.2.1 Argentina Intrapartum Monitoring Devices Market by Product Type
6.4.4.2.2 Argentina Intrapartum Monitoring Devices Market by Method
6.4.4.2.3 Argentina Intrapartum Monitoring Devices Market by End User
6.4.4.3 UAE Intrapartum Monitoring Devices Market
6.4.4.3.1 UAE Intrapartum Monitoring Devices Market by Product Type
6.4.4.3.2 UAE Intrapartum Monitoring Devices Market by Method
6.4.4.3.3 UAE Intrapartum Monitoring Devices Market by End User
6.4.4.4 Saudi Arabia Intrapartum Monitoring Devices Market
6.4.4.4.1 Saudi Arabia Intrapartum Monitoring Devices Market by Product Type
6.4.4.4.2 Saudi Arabia Intrapartum Monitoring Devices Market by Method
6.4.4.4.3 Saudi Arabia Intrapartum Monitoring Devices Market by End User
6.4.4.5 South Africa Intrapartum Monitoring Devices Market
6.4.4.5.1 South Africa Intrapartum Monitoring Devices Market by Product Type
6.4.4.5.2 South Africa Intrapartum Monitoring Devices Market by Method
6.4.4.5.3 South Africa Intrapartum Monitoring Devices Market by End User
6.4.4.6 Nigeria Intrapartum Monitoring Devices Market
6.4.4.6.1 Nigeria Intrapartum Monitoring Devices Market by Product Type
6.4.4.6.2 Nigeria Intrapartum Monitoring Devices Market by Method
6.4.4.6.3 Nigeria Intrapartum Monitoring Devices Market by End User
6.4.4.7 Rest of LAMEA Intrapartum Monitoring Devices Market
6.4.4.7.1 Rest of LAMEA Intrapartum Monitoring Devices Market by Product Type
6.4.4.7.2 Rest of LAMEA Intrapartum Monitoring Devices Market by Method
6.4.4.7.3 Rest of LAMEA Intrapartum Monitoring Devices Market by End User
Chapter 7. Company Profiles
7.1 Koninklijke Philips N.V.
7.1.1 Company Overview
7.1.2 Financial Analysis
7.1.3 Segmental and Regional Analysis
7.1.4 Research & Development Expense
7.1.5 Recent strategies and developments:
7.1.5.1 Product Launches and Product Expansions:
7.2 MindChild Medical, Inc.
7.2.1 Company Overview
7.2.2 Recent strategies and developments:
7.2.2.1 Partnerships, Collaborations, and Agreements:
7.3 Arjo AB (Huntleigh Healthcare ltd.)
7.3.1 Company Overview
7.3.2 Financial Analysis
7.3.3 Segmental Analysis
7.3.4 Research & Development Expenses
7.4 Stalwart Meditech Private Limited
7.4.1 Company Overview
7.5 MedGyn Products, Inc.
7.5.1 Company Overview
7.6 Cardinal Health, Inc.
7.6.1 Company Overview
7.6.2 Financial Analysis
7.6.3 Segmental and Regional Analysis
7.7 GE HealthCare Technologies, Inc.
7.7.1 Company Overview
7.8 CooperVision, Inc. (THE COOPER COMPANIES, INC.)
7.8.1 Company Overview
7.8.2 Financial Analysis
7.8.3 Segmental and Regional Analysis
7.8.4 Research & Development Expenses
7.9 Olympus Corporation
7.9.1 Company Overview
7.9.2 Financial Analysis
7.9.3 Segmental and Regional Analysis
Companies Mentioned
- Koninklijke Philips N.V.
- MindChild Medical, Inc.
- Arjo AB (Huntleigh Healthcare ltd.)
- Stalwart Meditech Private Limited
- MedGyn Products, Inc.
- Cardinal Health, Inc.
- GE HealthCare Technologies, Inc.
- CooperVision, Inc. (THE COOPER COMPANIES, INC.)
- Olympus Corporation
Methodology

LOADING...