Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The market’s growth is driven by progress in genetic research, which has enabled the development of advanced therapies such as gene therapy and precision medicine. Regulatory incentives like the Orphan Drug Act in the U.S. and similar frameworks globally have encouraged pharmaceutical companies to invest in this sector. Additionally, increasing awareness through patient advocacy and healthcare organizations has elevated rare diseases as a priority, spurring funding and innovation. Together, these factors are fostering a supportive environment for therapeutic advancements and improving outcomes for individuals affected by rare diseases.
Key Market Drivers
Rising Number of Patients with Rare Diseases
The growing recognition of rare diseases, which number around 7,000 globally, is a significant driver for the market. Conditions such as cystic fibrosis, cat eye syndrome, and numerous rare cancers and metabolic disorders often lack effective treatments. According to the Indian Organization for Rare Diseases, a vast majority of these conditions still do not have targeted therapies.Similarly, the World Economic Forum reported in 2020 that approved treatments are available for only about 5% of known rare diseases, underscoring an enormous unmet medical need. In Europe, rare diseases affect approximately 6% to 8% of the population, amounting to nearly 30 million people. However, the individual rarity of each condition leads to limited research investment, late diagnoses, and inadequate treatment infrastructure. This "rarity paradox" highlights the mismatch between collective prevalence and resource allocation, emphasizing the need for policy reform, cross-border research collaboration, and incentivized innovation to address therapeutic gaps for this large patient population.
Key Market Challenges
Accurate Diagnosis
Accurate diagnosis remains a major barrier in rare disease management. For many patients, securing a definitive diagnosis can take up to five years due to the subtle and often non-specific nature of symptoms. Physicians may struggle to identify rare conditions, particularly those they have never encountered. In such cases, comprehensive genetic testing may be required to detect disease-related mutations. However, interpreting complex genetic data poses challenges, especially in the absence of specialists. To improve diagnostic accuracy, patients benefit most from referrals to clinical geneticists or healthcare providers with experience in managing rare diseases. Delays in diagnosis hinder timely treatment initiation and reduce eligibility for clinical trials, making early identification critical for improving patient outcomes and accelerating research.Key Market Trends
Growing Product Innovation
The growing demand for rare disease treatments has spurred significant product innovation. Leading pharmaceutical companies are accelerating the development and global rollout of new therapies. For example, in February 2023, Sanofi announced plans to launch two orphan drugs in India: Nexviazyme (avalglucosidase alfa) for Pompe disease and Xenpozyme (olipudase alfa) for Niemann-Pick disease (ASMD). Both drugs are already approved in markets such as the U.S., EU, UK, Japan, and Australia, and have received waivers for Phase III and IV trials in India. This trend highlights a broader industry shift toward expanding access to specialized treatments in emerging markets, supported by regulatory flexibility and increased investment in research. As more therapies secure global approvals, innovation continues to reshape the landscape, offering hope for patients with previously untreatable conditions.Key Market Players
- Novartis AG
- AstraZeneca PLC
- Pfizer Inc.
- Sanofi SA
- AbbVie Inc
- Bristol-Myers Squibb Co
- Bayer AG
- F Hoffmann-La Roche Ltd.
- Amgen Inc
- Eisai Co Ltd
- Novo Nordisk A/S
Report Scope:
In this report, the Global Rare Disease Therapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Rare Disease Therapeutics Market, By Therapeutic Area:
- Hematologic Diseases
- Cancer
- Infectious Diseases
- Cardiovascular Diseases
- Metabolic Diseases
- Endocrine Diseases
- Musculoskeletal Diseases
- Others
Rare Disease Therapeutics Market, By Route of Administration:
- Injectable
- Oral
- Others
Rare Disease Therapeutics Market, By Drug Type:
- Biologics
- Biosimilar
- Small Molecules
Rare Disease Therapeutics Market, By Distribution Channel:
- Specialty Pharmacies
- Hospital Pharmacies
- Online Pharmacies
Rare Disease Therapeutics Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Rare Disease Therapeutics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Novartis AG
- AstraZeneca PLC
- Pfizer Inc.
- Sanofi SA
- AbbVie Inc
- Bristol-Myers Squibb Co
- Bayer AG
- F Hoffmann-La Roche Ltd.
- Amgen Inc
- Eisai Co Ltd
- Novo Nordisk A/S
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | June 2025 |
| Forecast Period | 2024 - 2030 |
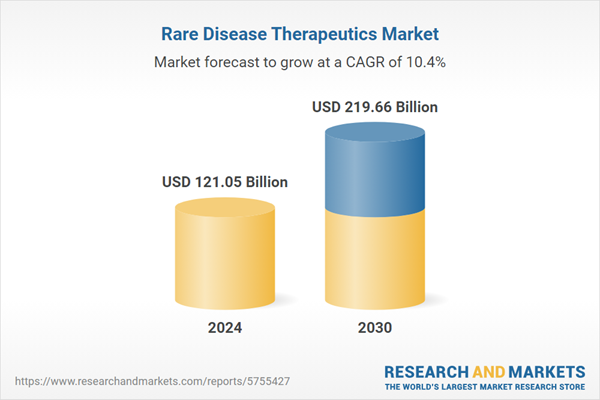
| Estimated Market Value ( USD | $ 121.05 Billion |
| Forecasted Market Value ( USD | $ 219.66 Billion |
| Compound Annual Growth Rate | 10.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |