Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Additionally, growing demand for the best treatment with low cost, along with growing geriatric population have significantly increased the demand for vulvovaginal candidiasis treatment across different parts of the globe. Additionally, increasing prevalence of chronic diseases like cancer, cardiovascular diseases, neurological diseases, and diabetes across the globe is further expected to increase the demand for vulvovaginal candidiasis treatment, thereby fuelling the market growth. Besides, growing awareness on maintaining hygiene and benefits of using new treatments with lower cost is further expected to support the vulvovaginal candidiasis treatment market during the forecast period.
Furthermore, increasing usage of broad-spectrum antibiotic such as Fluconazole because of yeast infection is further expected to increase the demand for vulvovaginal candidiasis treatment, thereby supporting the market growth. It is the second most common type of vaginal infection after bacterial vaginal infections, in the United States. An estimated 1.4 million outpatient visits for vaginal candidiasis occur annually.
Key Market Drivers
Rising Prevalence of Vulvovaginal Candidiasis and Recurrent Infections
One of the primary drivers of growth in the global VVC treatment market is the rising prevalence of both acute and recurrent vulvovaginal candidiasis infections. Vulvovaginal candidiasis is caused predominantly by Candida albicans, and affects a large proportion of women worldwide, especially those of reproductive age. According to the Centers for Disease Control and Prevention (CDC), approximately 75% of all women will experience at least one episode of VVC during their lifetime, and nearly 40-45% of these individuals will experience two or more episodes.Around 5-8% of women are affected by recurrent vulvovaginal candidiasis (RVVC), which is defined as four or more symptomatic episodes within a year. Several factors contribute to this high prevalence: increased antibiotic use, hormonal changes (due to pregnancy or contraceptives), diabetes, immunosuppression (including HIV/AIDS), and the growing use of immunosuppressive medications. The World Health Organization (WHO) notes that nearly 37.7 million people globally were living with HIV as of 2023, a key risk factor for fungal infections such as VVC.
The growing global awareness surrounding women's reproductive health, increased healthcare access, and proactive screening campaigns are further driving diagnosis rates, prompting a higher demand for effective antifungal therapies. Moreover, the global emphasis on sexual health and infection control, particularly in developing regions, has led to increased funding and outreach efforts aimed at early detection and treatment of fungal infections. Collectively, these trends underscore a growing need for both over the counter (OTC) and prescription treatments, fueling market expansion.
Key Market Challenges
Antifungal Resistance and Limited Treatment Efficacy
A significant challenge to the VVC treatment market is the increasing resistance to conventional antifungal drugs, particularly azoles. Repeated and often inappropriate use of azole antifungals such as fluconazole has led to decreased efficacy in many patients, especially those with recurrent vulvovaginal candidiasis (RVVC). Drug-resistant Candida species, including Candida glabrata and Candida auris, are becoming more prevalent and are often harder to treat. The World Health Organization (WHO), in its 2023 publication on fungal priority pathogens, classified Candida auris as a critical priority pathogen due to its high resistance profile, global spread, and association with severe healthcare outbreaks. The WHO has also recognized the urgent need for new antifungal drug development to address growing treatment gaps and antimicrobial resistance (AMR).Furthermore, research by the Centers for Disease Control and Prevention (CDC) reveals that fluconazole resistance among Candida strains has increased steadily in recent years, complicating treatment decisions for physicians and leading to higher rates of treatment failure. This resistance is compounded by patient non-compliance, self-medication, and lack of definitive diagnostics, especially in low-resource settings. Addressing antifungal resistance requires substantial investment in surveillance, diagnostics, and antimicrobial stewardship programs. However, in many countries, such efforts remain underfunded or fragmented, hindering the timely management of resistant infections and posing a long-term threat to treatment efficacy and patient outcomes.
Key Market Trends
Shift Toward Novel and Non-Azole Antifungal Therapies
A significant trend in the VVC treatment market is the development and growing preference for non-azole antifungal therapies, particularly in cases of recurrent or drug-resistant infections. Traditional azole-based medications (like fluconazole, clotrimazole, and miconazole) have long been the mainstay for treating VVC. However, emerging drug resistance and the limited efficacy of azoles in recurrent infections have necessitated the development of novel antifungal agents. The shift is supported by increased research and regulatory momentum. For instance, the U.S. FDA approved ibrexafungerp, a novel triterpenoid antifungal agent, for the treatment of VVC in 2021. This new class of antifungals works differently from azoles, targeting the fungal cell wall rather than its membrane, offering a promising alternative for patients with azole-resistant candidiasis or intolerance to existing medications.Government research institutions and funding agencies are also prioritizing antimicrobial resistance (AMR) and fungal infections. The CDC’s Antibiotic Resistance Threats Report (2023) includes Candida species as serious threats, especially Candida auris, which is resistant to multiple antifungal drugs. As part of its response, the U.S. government is supporting antifungal drug development under initiatives such as the National Action Plan for Combating Antibiotic-Resistant Bacteria (CARB). This trend is likely to grow stronger as more patients and physicians seek effective treatment options for recurrent infections. Moreover, the pharmaceutical industry is investing in oral and topical agents that offer better bioavailability, fewer side effects, and resistance management - driving innovation in the market landscape.
Key Market Players
- Astellas Pharma Inc.
- Mycovia Pharmaceuticals, Inc.
- Basilea Pharmaceutica Ltd.
- Scynexis, Inc.
- Grupo Ferrer Internacional, S.A.
- Pfizer, Inc.
- Pacgen Life Science Corporation
- NovaDigm Therapeutics, Inc.
- Cidara Therapeutics, Inc.
- Amplyx Pharmaceuticals Inc.
Report Scope:
In this report, the Global Vulvovaginal Candidiasis Treatment Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Vulvovaginal Candidiasis Treatment Market, By Drug Class:
- Clotrimazole
- Nystatin
- Fluconazole
- Ketoconazole
- Terbinafine
- Terconazole
- Others
Vulvovaginal Candidiasis Treatment Market, By Route of Administration:
- Oral
- Intravenous
- Topical
Vulvovaginal Candidiasis Treatment Market, By Distribution Channel:
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Vulvovaginal Candidiasis Treatment Market, By Region:
- North America
- United States
- Mexico
- Canada
- Europe
- France
- Germany
- United Kingdom
- Italy
- Spain
- Asia-Pacific
- China
- India
- South Korea
- Japan
- Australia
- South America
- Brazil
- Argentina
- Colombia
- Middle East and Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Vulvovaginal Candidiasis Treatment Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
Table of Contents
Companies Mentioned
- Astellas Pharma Inc.
- Mycovia Pharmaceuticals, Inc.
- Basilea Pharmaceutica Ltd.
- Scynexis, Inc.
- Grupo Ferrer Internacional, S.A.
- Pfizer, Inc.
- Pacgen Life Science Corporation
- NovaDigm Therapeutics, Inc.
- Cidara Therapeutics, Inc.
- Amplyx Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | August 2025 |
| Forecast Period | 2024 - 2030 |
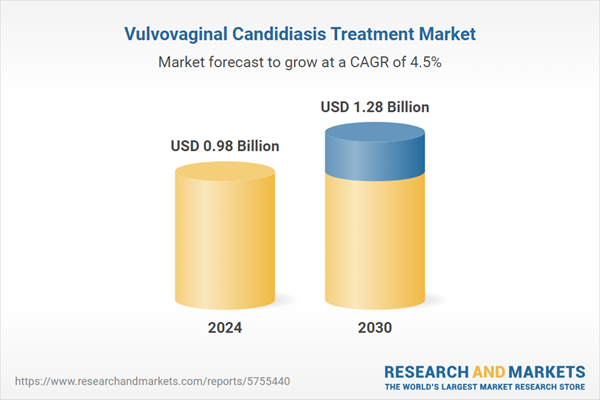
| Estimated Market Value ( USD | $ 0.98 Billion |
| Forecasted Market Value ( USD | $ 1.28 Billion |
| Compound Annual Growth Rate | 4.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |