The COVID-19 outbreak put an immense burden on the healthcare infrastructure to develop advanced treatments and vaccines. Owing to the high specificity and reliability of next-generation antibody therapeutics, monoclonal antibodies (mAbs) emerged as powerful tools to treat and detect numerous diseases, including SARS and CoV-2, which resulted in the evolution of the next-generation antibody therapeutics sector. As per the article published in January 2022 in the Journal of Biomedical Science, many researchers have started to urgently develop antibody-based assays for the detection of SARS-CoV-2 and Ab therapeutics for use as COVID-19 treatment agents. For instance, in September 2022, AstraZeneca's Evusheld (tixagevimab and cilgavimab, formerly AZD7442), a long-acting antibody combination, has been recommended for marketing authorization in the European Union for the treatment of adults and adolescents, aged 12 years and older, weighing at least 40 kg, with COVID19 who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID19. Hence, the COVID-19 pandemic was anticipated to positively impact the next-generation antibody therapeutics market in the coming years.
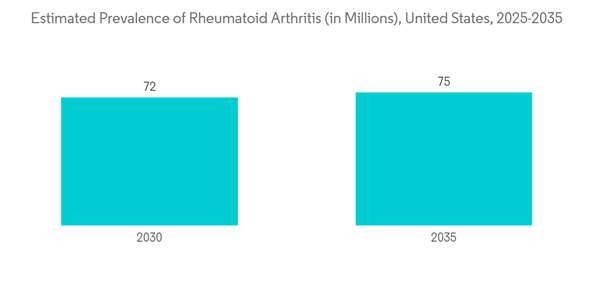
The major factor attributing to the growth of the market is the increase in the prevalence of different chronic diseases such as rheumatoid arthritis, inflammatory bowel disorder, Crohn’s disease, and cancer. According to the research published by Medscape in January 2022, the annual incidence rate of rheumatoid arthritis is approximately 3 cases per 10,000 people, and the prevalence rate is approximately 1% globally. Furthermore, the American Cancer Society published the statistics in January 2021, estimating more than 1.9 million new cancer cases to be diagnosed and 609,360 cancer deaths in the United States in 2022. Due to their enhanced efficacy, increased safety, and ability to deliver targeted treatment, next-generation antibody therapies are primarily used to treat chronic diseases, including cancer and autoimmune disorders. Thus, it is anticipated that the studied market would expand in a lucrative way due to the rising prevalence of chronic diseases and rare disorders, where next-generation antibodies serve as the predominant therapy.
Leading biotechnology and pharmaceutical corporations are increasingly funding the creation of new therapeutic antibodies to fight inflammatory illnesses, cancer, and autoimmune disorders. For instance, in November 2022, Seagen announced the US Food and Drug Administration's (FDA) approval of a new indication for brentuximab vedotin, ADCETRIS, for children with previously untreated high-risk Hodgkin lymphoma. In November 2022, ImmunoGen announced that the U.S. FDA had granted accelerated approval for ELAHERE (mirvetuximab soravtansine-gynx) for the treatment of platinum-resistant ovarian cancer in adults. Additionally, in July 2022, F-star Therapeutics announced a license agreement to grant Takeda a worldwide, exclusive, royalty-bearing license to research, develop, and commercialize a bispecific next-generation antibody against an immuno-oncology target using F-star’s proprietary Fcab and mAb2 platforms. In March 2022, Bayer announced its plans to spend EUR 2 billion over the next three years to expand its next-gen manufacturing capabilities.
Therefore, the studied market is likely to witness growth over the forecast period due to several factors, such as growing advancements in monoclonal antibody technology, rising incidences of chronic disorders, and increasing initiatives for the discovery and development of next-generation antibody therapeutics. However, strict regulations on the approval of these therapeutics, coupled with the inflated costs of the next generation of antibody therapeutics, are expected to hinder market growth during the study period.
Key Market Trends
Antibody-Drug Conjugates Segment is Expected to Hold the Largest Market Share in the Next-Generation Antibody Therapeutics Market
Antibody-Drug Conjugates (ADC) are a significant advancement in next-generation antibody therapeutics because ADC technology combines the cytotoxic potential of chemotherapy with the advantageous properties of antibodies, resulting in ADCs with high specificity and efficiency.Next-generation antibody therapies for the treatment of cancer are mostly manufactured using ADC technology. ADCs are manufactured by linking an anti-cancer drug or another therapeutic substance to an antibody or antibody fragment. For instance, according to research published in June 2021 in The Oncologist, sacituzumab govitecan, an antibody-drug conjugate composed of a humanized anti-Trop-2 antibody conjugated to SN38, proved to be an effective and generally well-tolerated agent that represents a promising novel therapy for patients with metastatic triple-negative breast cancer.The high demand for antibody-based cancer therapy, along with the increasing incidence of breast cancer globally, new technological advancements, and a rapid rise in the number of these conjugates under clinical trials, will likely drive the antibody-drug conjugates (ADC) segment to dominate the market during the forecast period. According to the statistics reported by BreastCancer.org in October 2022, breast cancer is currently the most common cancer globally, accounting for 12.5% of all new annual cancer cases worldwide. Similarly, the American Cancer Society published the study in October 2022, reporting an estimated 287,850 new cases of invasive breast cancer expected to be diagnosed in women in the U.S., along with 51,400 new cases of non-invasive (in situ) breast cancer annually. Due to these strong demands, the market will grow quickly over the next few years.
The rising demand for these antibody therapeutics has resulted in the rapid increase of new drug approvals for ADCs. Several players are actively involved in the development of next-generation antibody therapeutics using ADC technology through various strategies. For instance, in February 2022, Mersana Therapeutics Inc. announced its partnership with Janssen Pharmaceutical Companies for the research and development of novel ADCs to treat numerous cancers. Additionally, in June 2022, ImmunoGen Inc. announced a multi-year research collaboration with Oxford BioTherapeutics to develop novel antibody-drug conjugates. These kinds of collaborations are fueling the growth of the segment and propelling the next-generation antibody therapeutics market's revenue in the forecast period.
North America is Expected to Dominate the Next-Generation Antibody Therapeutics Market
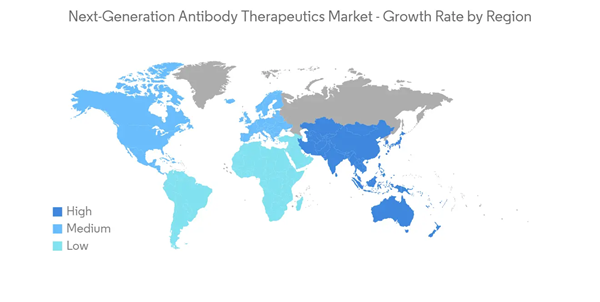
North America is anticipated to dominate the overall next-generation antibody therapeutics market throughout the forecast period. The dominance is due to several factors, such as a rise in auto-immune diseases, increased healthcare expenditure, new product launches, and continuous growth in R&D activities with the rise in the application of these therapeutics in the treatment of various disorders.Further, the growing incidence of chronic disorders such as cancer and asthma is driving the next-generation antibody therapeutics market. According to a report published in September 2022 by the Asthma and Allergy Foundation of America, approximately 25 million people in the United States have asthma each year, accounting for one in every thirteen Americans.Further, as per the report published by Breast Cancer.Org in October 2022, there are more than 3.8 million women in the U.S. with a history of breast cancer as of January 2022. Thus, the escalation in the prevalence of chronic diseases in the region is driving the size of the sector.
Moreover, the presence of significant companies in the region, the rapid approval of next-generation antibody therapies, and the availability of reputable research centers all contribute to the market's expansion. According to the article published in November 2022 in Silicon Republic, as of 2021, the U.S. FDA has approved its 100th monoclonal antibody therapeutic. For instance, in August 2022, AstraZeneca and Daiichi Sankyo’s Enhertu (trastuzumab deruxtecan) will be approved in the United States for the treatment of adult patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received prior chemotherapy in the metastatic setting or developed disease recurrence during or within six months of completing adjuvant chemotherapy. In December 2022, Pyxis Oncology, Inc. received clearance from the U.S. FDA to initiate Phase 1 clinical trials for its Investigational New Drug (IND) application of PYX-201, a novel antibody-drug conjugate (ADC) product candidate. The market is anticipated to advance in the next few years, driven by several approvals and many products currently in the pipeline. Furthermore, the presence of advanced healthcare infrastructure and well-established direct reimbursement policies is also expected to contribute significantly to market revenue throughout the forecast period.
Competitive Landscape
The market for next-generation antibody therapeutics is moderately competitive and consists of several major players. In terms of market share, a few of the major players are currently dominating the market. Some prominent players are acquiring other companies to consolidate their market positions across the globe, while others are launching new products. Some of the companies that are currently dominating the market are Amgen Inc., AstraZeneca Plc., Bayer AG, Biogen, Bristol-Myers Squibb Company, GlaxoSmithKline Plc., F. Hoffmann-La Roche Ltd., ImmunoGen Inc., Kyowa Hakko Kirin Co., Ltd., Pfizer Inc., Seagen Inc., and Xencor, Inc., among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Amgen Inc.
- AstraZeneca Plc.
- Bayer AG
- Biogen
- Bristol-Myers Squibb Company
- GlaxoSmithKline Plc.
- F. Hoffmann-La Roche Ltd
- ImmunoGen Inc.
- Kyowa Hakko Kirin Co., Ltd.
- Pfizer Inc.
- Seagen Inc.
- Xencor, Inc.