The COVID-19 pandemic had a substantial impact on patients with any disease, the prevalence of allergic diseases showed heterogeneous patterns during the COVID-19 pandemic, and epinephrine autoinjectors helped the patients to combat its effect. The rising adoption of epinephrine autoinjectors during the COVID due to severe allergic reactions reported after the administration of COVID-19 vaccines is expected to contribute to the growth of the market during the pandemic period. For instance, the FDA's October 2020 update and the Centers for Disease Control and Prevention (CDC) guidance entitled ‘Emergency Use Authorization for Vaccines to Prevent COVID-19’s state that if an acute anaphylactic reaction occurs after the administration of a COVID-19 vaccine, the administering facility should always have at least 3 doses of age-appropriate epinephrine available, as well as the ability to quickly obtain additional doses to replenish supplies after epinephrine runs out.
The rise in availability of several versions of products, innovative drug administration techniques that match the demands of patients, increasing awareness about self-administration, and adoption of more expensive auto-injectors over traditional injecting methods, as well as enhanced healthcare infrastructure, are among the major factors driving the growth of the studied market. Furthermore, favorable reimbursement policies in developed countries are anticipated to fuel the growth in the market.
According to World Health Organization reports, over 16 billion injections were administered annually worldwide in 2021, stimulated by the increasing number of injections administered boosting the autoinjector market. As a result, the global epinephrine autoinjector market is anticipated to develop in the future due to the convenience of use and disposal of drug injectors and the acceptance of autoinjectors, which are positioned to accelerate market expansion for the researched industry throughout the projected period.
In addition, new product launches and strategic activities, such as mergers/acquisitions, recent developments, joint ventures, collaborations, and partnerships by major players in the market are positively affecting the growth of the studied market. For instance, Catalent scheduled to manufacture a new batch of SYMJEPI in November 2022, for Adamis Pharmaceuticals Co. in Belgium, anticipating the relaunch of SYMJEPI and commercial availability before the end of the first quarter of 2023.
Therefore, owing to the aforementioned factors the studied market is anticipated to witness growth over the analysis period. However, regulatory constraints and the inflated cost of epinephrine autoinjectors are likely to impede the market growth.
Epinephrine Autoinjector Market Trends
0.3 mg Dosage is Expected to Hold a Significant Share Over the Forecast Period
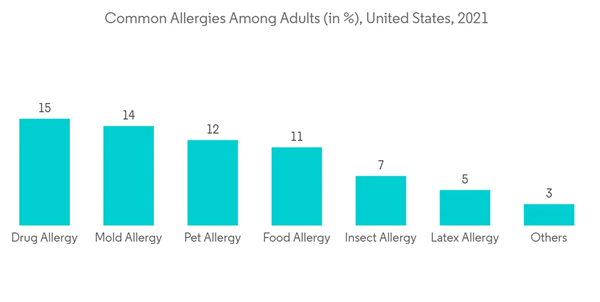
Several social and psychological variables contribute to the high frequency of anaphylaxis in teen and adult populations. Healthcare professionals recommend that patients utilize epinephrine auto-injectors at a safe dose if they are 12 years of age or older (often 0.3 mg). Numerous guidelines, including RCUK 2008/2021 valid in the United Kingdom, EAACI 2014/2021 applicable in the European region, and World Allergy Organization Anaphylaxis Guidance 2020 applicable in nearly 100 countries around the globe reduced the dosing regimen to age groups based on what is regarded to be safe and practical to prepare and inject in an emergency. Further, these guidelines stated to provide 0.3 mg of epinephrine intramuscularly to children aged 6 to 12 in order as an advisable dosage to ensure their safety and efficacy.According to several studies, most cases of food allergies, including anaphylaxis, happen at schools or daycare facilities. Epinephrine is suggested as a first-line therapy option in the guidelines of the National Institute of Allergy and Infectious Diseases. The rising recommendation of high doses for adult patients is promoting the segment's expansion. Players in the business are also concentrating on offering generic goods at low prices. For instance, in November 2022, Amphastar Pharmaceuticals indicated that their Epinephrine sales increased by USD 3.3 million for the three months ended September 30, 2022, due to an increase in average selling price, with the remainder of the increase due to increased unit volumes as a result of competitor shortages.
As a result, there is a growing demand for the development of new therapeutics to control anaphylactic reactions. For instance, in October 2022, ARS Pharmaceuticals, Inc. announced that FDA has accepted for review ARS’ New Drug Application (NDA) for ‘neffy’, which is the first non-injectable treatment for the emergency treatment of allergic reactions (type I) including anaphylaxis in adults and children. Furthermore, continuous product launches by major players in the market are positively affecting the growth of the segment. For instance, in January 2022, Massachusetts-based Windgap Medical reported raising USD 39 million and indicated its plans in developing products based on its Andipen autoinjector technology.
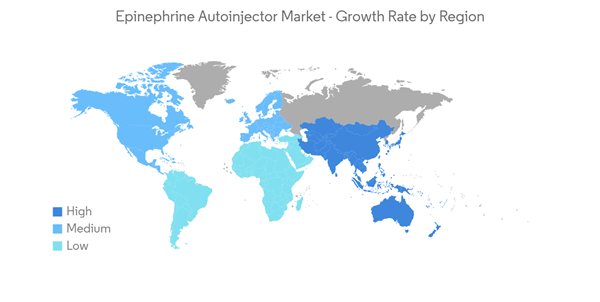
North America is Expected to Dominate the Epinephrine Autoinjector Market
North America is expected to hold a significant share of the global disposable auto-injectors market during the forecast period. Factors, such as the easy availability of products and the launch of generic substitutes as well as the rising incidence of anaphylaxis, such as allergies caused by food items, food additives, latex, and dust, coupled with better access to treatment for anaphylaxis boost the market in the region.According to the National Library of Medicine, an article published in 2021 in PubMed, between 1.6% and 5.1% of US citizens annually is estimated to have experienced anaphylaxis or suffer from this condition. Also, in March 2022, the Asthma and Allergy Foundation of America, about 7.7% of adults and 7.2% of children have been diagnosed with seasonal allergic rhinitis. The growing need for epinephrine auto-injectors for quick emergency response is a major factor propelling market expansion.
Key product launches, the high concentration of market players, and the manufacturer's presence in the United States are some of the factors driving the growth of the epinephrine autoinjector market in the country. For instance, in June 2021, Alerje Inc., a Detroit-based food allergy management firm developed a smartphone case with an auto-injector that quickly delivers epinephrine for severe allergic reactions. The Alerje smartphone app will immediately alter the patients' support network if a dosage is taken out of the case. Again, in August 2022 Sanofi, announced FDA approval for Auvi-Q, its first voice-guided epinephrine auto-injector for patients with life-threatening allergies., granting the company a license to commercialize Auvi-Q from Intelliject Inc. in North America while retaining commercialization rights for the rest of the world.
Epinephrine Autoinjector Market Competitor Analysis
The epinephrine autoinjector market is consolidated in nature with the presence of a few established vendors holding the majority of the shares and operating globally. The competitive landscape includes an analysis of a few international as well as local companies which hold market shares and are well known, including Adamis Pharmaceuticals Corporation, Alk-Abello A/S, Amneal Pharmaceuticals, Mylan NV, Antares Pharma, Pfizer Inc., Teva Pharmaceutical Industries Ltd., Bausch & Lomb Incorporated, Sanofi SA, Novartis International AG, among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Adamis Pharmaceuticals Corporation
- Alk-Abello A/S
- Amneal Pharmaceuticals
- Antares Pharma
- Bausch & Lomb Inc.
- Kaleo Inc.
- Mylan NV
- Pfizer Inc
- Sandoz
- Sanofi SA
- Teva Pharmaceutical Industries Ltd