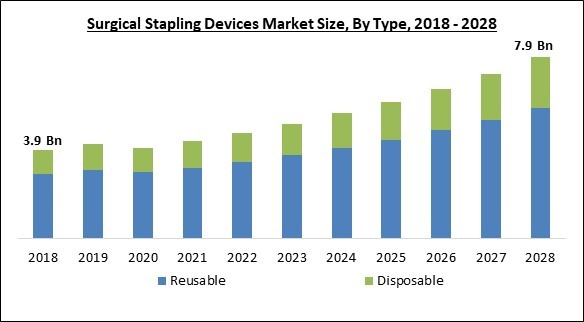
The Global Surgical Stapling Devices Market size is expected to reach $7.9 billion by 2028, rising at a market growth of 9.4% CAGR during the forecast period.
Surgical stapling devices apply surgical staples instead of sutures to heal skin wounds or keep tissue together. When operating time is an issue and aesthetic closure is not a top priority, surgical staples may help close injuries quickly. There are now three main categories of mechanical staplers for open and endoscopic surgery: linear, circular, and endoscopic surgical staplers.
Several novel surgical equipment and gadgets are now available because of technological advancements in various scientific fields. To enhance patient outcomes, surgeons can create novel surgical procedures due to the constant introduction of new instruments and the ongoing technological advances in already-existing technologies.
In many instances, surgeons may need to learn the scientific or clinical foundation for the best use of these technologies or how to benefit from any particular advantages a specific technology may have. So, even if the devices work well, surgeons may often depend on their expertise, judgment, or anecdotal evidence for better results.
Equipment that is often employed during surgical operations and is also undergoing a nearly continual state of technological evolution is the surgical stapler. While these tools are adaptable and practical, there have been well-reported instances of staple line leaks that resulted in postoperative difficulties, many of which were caused by problems unrelated to ischemia.
In operations, particularly challenging ones like gynecologic, gastrointestinal, & bariatric procedures, surgical stapling instruments are becoming more and more crucial. In addition, the use of surgical stapling devices facilitates the development of novel surgical techniques, the alteration of existing procedures, and the enhancement of clinical results. The increasing demand for less invasive treatments globally drives the expansion of the surgical stapling devices market.
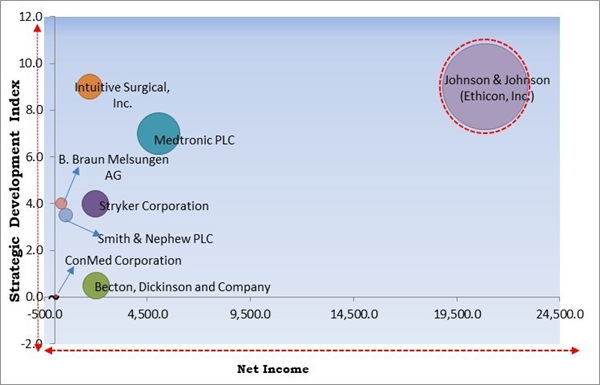
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; Johnson & Johnson (Ethicon, Inc.) is the forerunner in the Surgical Stapling Devices Market. Companies such as Intuitive Surgical, Inc., Medtronic PLC, and Stryker Corporation are some of the key innovators in Surgical Stapling Devices Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Stryker Corporation, Medtronic PLC, BioPro, Inc., ConMed Corporation, Smith & Nephew PLC, Intuitive Surgical, Inc., Johnson & Johnson (Ethicon, Inc.), B. Braun Melsungen AG, Becton, Dickinson and Company and Meril Life Science Private Limited.
Surgical stapling devices apply surgical staples instead of sutures to heal skin wounds or keep tissue together. When operating time is an issue and aesthetic closure is not a top priority, surgical staples may help close injuries quickly. There are now three main categories of mechanical staplers for open and endoscopic surgery: linear, circular, and endoscopic surgical staplers.
Several novel surgical equipment and gadgets are now available because of technological advancements in various scientific fields. To enhance patient outcomes, surgeons can create novel surgical procedures due to the constant introduction of new instruments and the ongoing technological advances in already-existing technologies.
In many instances, surgeons may need to learn the scientific or clinical foundation for the best use of these technologies or how to benefit from any particular advantages a specific technology may have. So, even if the devices work well, surgeons may often depend on their expertise, judgment, or anecdotal evidence for better results.
Equipment that is often employed during surgical operations and is also undergoing a nearly continual state of technological evolution is the surgical stapler. While these tools are adaptable and practical, there have been well-reported instances of staple line leaks that resulted in postoperative difficulties, many of which were caused by problems unrelated to ischemia.
In operations, particularly challenging ones like gynecologic, gastrointestinal, & bariatric procedures, surgical stapling instruments are becoming more and more crucial. In addition, the use of surgical stapling devices facilitates the development of novel surgical techniques, the alteration of existing procedures, and the enhancement of clinical results. The increasing demand for less invasive treatments globally drives the expansion of the surgical stapling devices market.
COVID-19 Impact Analysis
The lockdown compelled many hospitals to stop their outpatient departments for situations other than emergencies, which had a significant impact on the market for surgical stapling devices. Social exclusion, population control, and restricted clinic access substantially influenced the market. However, the market is anticipated to grow significantly after the lockdown restrictions are lifted as significant manufacturers are concentrating on introducing technologically cutting-edge items to increase their market share. Also, with production getting back on track, and the resume in surgical procedures which is increasing the demand, the surgical stapling devices market will start to expand again after the pandemic.Market Growth Factors
The technological betterment of surgical stapling devices
Technological developments in surgical staples and related staples have significantly impacted the acceptance of surgical stapling devices by healthcare practitioners. With the introduction of powered surgical staplers, the time needed to seal the wound after surgery has decreased, minimizing problems and blood loss. In clinical testing, the powered surgical stapler performed better in the speed of healing and decreased discomfort. Hence, it is anticipated that the technical benefits offered by surgical stapling devices will drive market growth.Rising prevalence of chronic diseases
Due to the rise in chronic illnesses like cancer during the last several years, there has also been an increase in the number of visits to ambulatory surgical facilities. Moreover, it is predicted that the market for surgical staplers will grow throughout the projection years due to the rise in laparoscopic treatments chosen by patients. The decreasing cost, shorter hospital stays, and increasing popularity of laparoscopic operations are all due to these factors. Hence with the high prevalence of chronic cases and the rising popularity of laparoscopic operations, the market growth for surgical stapling devices will surge.Market Restraining Factors
Common issues with surgical staplers
Opening of the staple line at a wound site, faulty staples that result in insufficient sealing, defective or jammed staplers, failing to shoot a staple from a stapler, staples that were improperly positioned on the wrong tissue spot using a staple that is the incorrect size for a particular application are some of the common issues associated with the usage of stapling devices. These frequent issues may result in surgical staple wounds, lengthening healing time and exposing the patient to higher medical and rehabilitation expenses. These issues with the stapling devices are expected to restrict their market expansion.Product Type Outlook
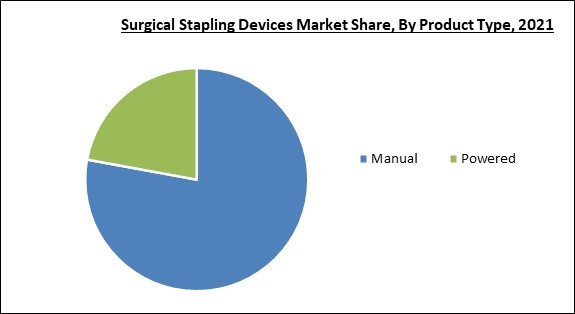
Based on product type, the surgical stapling devices market is segmented into powered and manual. The powered segment acquired a significant revenue share in the surgical stapling devices market in 2021. This is due to their simplicity of wound closure without requiring physical effort. Medical professionals now have more options due to the availability of powered surgical stapling devices in various sizes. In addition, the escalating technical advancements, product launches, and firms' increased emphasis on developing powered stapling devices are among other factors which are anticipated to boost the segment growth.Type Outlook
On the basis of type, the surgical stapling devices market is divided into disposable and reusable. The reusable segment held the highest revenue share in the surgical stapling devices market in 2021. This is because reusable surgical stapling instruments may be used on several patients after sterilizing. Due to the product's low cost, the category is expected to have a bigger share in developing countries. Furthermore, the introduction of surgical stapling devices combined with absorbable staplers is projected to boost the segment expansion in the projected period.End-use Outlook
By end-use, the surgical stapling devices market is classified into hospitals and ambulatory surgical centers. The hospitals segment witnessed the largest revenue share in the surgical stapling devices market in 2021. This is because hospitals are seeing a significant trend that points to a move towards clinical efficiency models, including robotic surgery. Minimally invasive procedures benefit from robotic surgery. The hospitals have well-trained staff and high-tech devices, which ensures a safe and successful procedure. Also, the hospital category is anticipated to maintain its dominance due to favorable reimbursement conditions for different methods during the projection period.Regional Outlook
Region-wise, the surgical stapling devices market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region registered the highest revenue share in the surgical stapling devices market in 2021. This is owing to the employment of cutting-edge technology and minimally invasive procedures with the rising number of total operations. In addition, the greatest obesity prevalence in the world, the local presence of multinational corporations, and the government's clearance for minimally invasive operations are other factors contributing to the large market share.The Cardinal Matrix - Surgical Stapling Devices Market Competition Analysis
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; Johnson & Johnson (Ethicon, Inc.) is the forerunner in the Surgical Stapling Devices Market. Companies such as Intuitive Surgical, Inc., Medtronic PLC, and Stryker Corporation are some of the key innovators in Surgical Stapling Devices Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Stryker Corporation, Medtronic PLC, BioPro, Inc., ConMed Corporation, Smith & Nephew PLC, Intuitive Surgical, Inc., Johnson & Johnson (Ethicon, Inc.), B. Braun Melsungen AG, Becton, Dickinson and Company and Meril Life Science Private Limited.
Strategies Deployed in Surgical Stapling Devices Market
Partnerships, Collaborations and Agreements:
- May-2022: Intuitive teamed up with Creo Medical Group, a UK-based medical device company. The collaboration includes optimizing Creo's products to make them consistent with Intuitive's robotic technology.
Product Launches and Product Expansions:
- Jun-2022: Ethicon, part of Johnson & Johnson introduced the ECHELON 3000 Stapler intended to use in resection, transection, and creation of anastomoses. The ECHELON 3000 is available in 45mm and 60mm staplers. The new stapler is a single-patient-use, sterile, that can cut and staple both at the same time.
- May-2022: Stryker introduced the EasyFuse Dynamic Compression System, which will be a part of Stryker's foot and ankle product portfolio. The new staple system is developed using a nickel-titanium alloy metal, known as nitinol. The EasyFuse Dynamic Compression System decreases complexity in surgeries. The newly launched product features, a wide staple bridge, an intuitively developed inserter, and sterile-packed instrumentation.
- Jun-2021: Intuitive unveiled SureForm, a robotic-assisted surgical stapler SureForm. The surgical stapler comes with SmartFire technology, an integrated software. The SureForm allows the surgeon to simultaneously fire the stapler from the console itself, and perform the RAS procedure. Further, the launch of the robot-assisted stapler reflects the company's advancement towards simplification of the surgery procedure.
- Mar-2021: Ethicon, part of Johnson & Johnson launched ECHELON+ Stapler equipped with Gripping Surface Technology (GST) Reloads. The new surgical stapler is developed to enhance staple line security, and lower complications. The stapler is equipped with a new motor with dynamic firing and a restructured ECHELON+Anvil.
- Dec-2020: Medtronic launched Tri-Staple EEA Circular Stapler in India. The 3-row circular stapler is equipped with Medtronic's Tri-Staple technology and has multiple height staples. The new circular stapler is intended to use in colorectal procedures. Additionally, the device is also equipped with enhanced tactile and audible feedback that allows clinicians to make a better informed decisions in the operating room.
- Feb-2020: Smith+Nephew introduced the CORI Surgical system. The new surgical systems are intended to enhance outcomes during knee arthroplasty, and also increase accuracy. Additionally, the CORI system is portable and easy to move from one theatre to the other.
Acquisitions and Mergers:
- Sep-2022: B. Braun took over Clik-FIX Catheter Securement Devices from Starboard Medical. The acquisition includes a Clik-FIX Peripheral catheter securement device, Clik-FIX Universal catheter securement device, a Clik-FIX Neonatal PICC catheter securement device, and Clik-FIX PICC/Central catheter securement device. The combination of the Clik-FIX portfolio and B.Braun's competence and deep knowledge enables the acquirer to fulfill the growing needs of its clients and further strengthens its market position in the IV therapy market.
- Nov-2020: Medtronic took over Medicrea, a France-based company primarily into developing, manufacturing, and marketing orthopedic implants intended for spinal surgery. This acquisition would reinforce Medtronic's position in personalized implants' AI-based prediction and planning capabilities.
Geographical Expansions:
- Nov-2022: Becton, Dickinson, and Company (BD) expanded its global footprint by setting up a new manufacturing facility in Tijuana, Mexico. The new facility would primarily manufacture devices and technologies. The establishment of the new facility demonstrates BD's devotion to Mexico and its strong relationship with communities across Mexico. This new plant would further simplify BD's operations by integrating a distribution center, a manufacturing site, and the transportation of products to the end consumer all in one place.
- Aug-2022: Stryker opened a new facility in Ireland. The new high-tech facility is a 156,000-square-foot facility, which would reinforce the company's market position in additive manufacturing.
- Jun-2022: Smith+Nephew opened a new manufacturing and R&D facility in Hull, United Kingdom. The new facility is intended for its advanced wound management franchise. The establishment of the new facility reflects the company's commitment to the UK and to building a leading market position in advanced wound management.
- Jun-2022: Stryker expanded its global footprint by setting up a new R&D facility in Haryana, India. The new 150,000-square-foot facility would advance innovation. Further, Stryker’s Global Technology Centre (SGTC) reinforces the company's potential to develop and design new products and solutions.
Trials and Approvals:
- Dec-2021: Intuitive received FDA approval for its SureForm 30. SureForm 30 is an 8 mm curved-tip stapler. The curved stapler is intended to use in thoracic, general, pediatric, urologic, and gynecologic surgeries.
Scope of the Study
By Type
- Reusable
- Disposable
By Product Type
- Manual
- Powered
By End-use
- Hospitals
- Ambulatory Surgical Centers
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Stryker Corporation
- Medtronic PLC
- BioPro, Inc.
- ConMed Corporation
- Smith & Nephew PLC
- Intuitive Surgical, Inc.
- Johnson & Johnson (Ethicon, Inc.)
- B.Braun Melsungen AG
- Becton, Dickinson and Company
- Meril Life Science Private Limited
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology
Chapter 2. Market Overview
Chapter 3. Competition Analysis - Global
Chapter 4. Global Surgical Stapling Devices Market by Type
Chapter 5. Global Surgical Stapling Devices Market by Product Type
Chapter 6. Global Surgical Stapling Devices Market by End-use
Chapter 7. Global Surgical Stapling Devices Market by Region
Chapter 8. Company Profiles
Companies Mentioned
- Stryker Corporation
- Medtronic PLC
- BioPro, Inc.
- ConMed Corporation
- Smith & Nephew PLC
- Intuitive Surgical, Inc.
- Johnson & Johnson (Ethicon, Inc.)
- B. Braun Melsungen AG
- Becton, Dickinson and Company
- Meril Life Science Private Limited