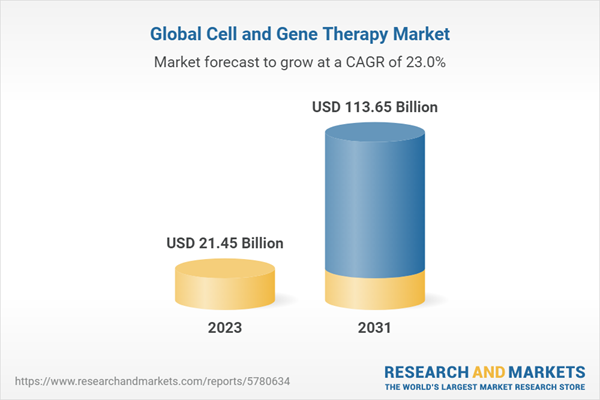
The global cell and gene therapy market size is projected to experience significant growth during the forecast period of 2023-2031, driven by the increasing prevalence of chronic diseases, advancements in gene editing technologies, and the rising demand for personalized medicine. The market is expected to grow at a CAGR of 23% during the forecast period to attain a value of USD 113.648 billion by 2031.
The CGT market is expected to experience significant growth in the coming years due to the increasing prevalence of chronic diseases, such as cancer and cardiovascular diseases, and the rising demand for innovative treatment options. Additionally, the increasing focus on personalized medicine and the adoption of advanced healthcare infrastructure are likely to create lucrative opportunities for the market players.
Furthermore, the increasing investments in research and development activities, coupled with the growing number of clinical trials, are expected to fuel the growth of the market. The adoption of favourable government policies and the increasing demand for better healthcare services in emerging economies are expected to further contribute to the growth of the market.
North America dominates the cell and gene therapy market, followed by Europe and the Asia Pacific. The high prevalence of chronic diseases, coupled with favourable government policies and the availability of advanced healthcare infrastructure, is driving the growth of the market in North America and Europe. The Asia Pacific region is expected to grow at the highest CAGR during the forecast period, owing to the increasing prevalence of chronic diseases and rising healthcare expenditure in the region.
The oncology segment dominates the cell and gene therapy market by therapeutic application, followed by cardiovascular diseases and central nervous system disorders. The increasing prevalence of cancer and the rising demand for innovative cancer treatments are driving the growth of the oncology segment. The cell therapy segment dominates the market by therapeutic modality, followed by gene therapy and tissue engineering. The increasing demand for personalized medicine and the growing number of clinical trials are driving the growth of the cell therapy segment.
Cell and Gene Therapy Market: Introduction
The global cell and gene therapy (CGT) market report provides an in-depth analysis of the market and its segments. Cell and gene therapies are advanced therapies that involve the use of living cells and genetic material to treat diseases. The report covers the market for CGT and provides a detailed analysis of the market size, growth, and trends.The CGT market is expected to experience significant growth in the coming years due to the increasing prevalence of chronic diseases, such as cancer and cardiovascular diseases, and the rising demand for innovative treatment options. Additionally, the increasing focus on personalized medicine and the adoption of advanced healthcare infrastructure are likely to create lucrative opportunities for the market players.
Furthermore, the increasing investments in research and development activities, coupled with the growing number of clinical trials, are expected to fuel the growth of the market. The adoption of favourable government policies and the increasing demand for better healthcare services in emerging economies are expected to further contribute to the growth of the market.
Cell and Gene Therapy Market - Application and Usage
Cell and gene therapy are advanced therapeutic techniques that use living cells or genetic material to treat a variety of diseases. They hold great promise in the treatment of various medical conditions, including genetic disorders, cancer, cardiovascular diseases, and autoimmune diseases.Here are some of the applications and uses of cell and gene therapy:
- Cancer Treatment: Cell and gene therapy offer a new approach to treating cancer by using modified immune cells to target and destroy cancer cells. This therapy has shown promising results in treating various types of cancer, such as leukaemia and lymphoma
- Genetic Disorders: Cell and gene therapy can correct genetic mutations that cause inherited disorders, such as sickle cell anaemia, haemophilia, and cystic fibrosis. This therapy involves inserting a normal copy of the gene into the patient's cells to restore their function
- Cardiovascular Diseases: Cell and gene therapy are being explored as a potential treatment for cardiovascular diseases such as heart failure and myocardial infarction. This therapy involves the use of stem cells to regenerate damaged heart tissue and improve heart function
- Autoimmune Diseases: Cell and gene therapy can modify immune cells to reduce the body's autoimmune response and treat autoimmune diseases such as multiple sclerosis and rheumatoid arthritis
- Ophthalmology: Cell and gene therapy are being explored as a treatment for inherited and acquired eye diseases, such as retinitis pigmentosa and age-related macular degeneration. This therapy involves the use of gene therapy to restore vision by repairing or replacing damaged cells in the retina
- Orthopaedics: Cell and gene therapy are being explored as a potential treatment for orthopaedic conditions such as osteoarthritis and bone fractures. This therapy involves the use of stem cells to regenerate damaged bone and cartilage tissue and promote healing
Cell And Gene Therapy Market Segmentations
The market can be categorised into type, indication, product type, end user, and major region.Market Breakup by Type
Cell Therapy Types
- Autologous Cell Therapy
- Autogenic Cell Therapy
- Ex-vivo Cell Therapy
- In-vivo Cell Therapy
Gene Therapy Types
- Somatic Cell Gene Therapy
- Germline Gene Therapy
- Ex-vivo Gene Therapy
- In-vivo Gene Therapy
Market Breakup by Indications
- Oncology
- Cardiology
- CNS
- Musculoskeletal
- Infectious Diseases
- Dermatology
- Endocrine, Metabolic, Genetic
- Immunology and Inflammation
- Ophthalmology
- Haematology
- Gastroenterology
- Others
Market Breakup by Product Type
- Yescarta
- Provenge
- Luxtura
- Kymriah
- Imlygic
- Gintuit
- MACI
- Laviv
- Gendicine
- Oncorine
- Neovasculgen
- Strimvelis
- Invossa
- Zolgenesma
- Tecartus
- Lisocel
- Zyntelego
- Others
Cell and Gene Therapy Market Breakup by End User
- Hospitals
- Ambulatory Surgical Centres
- Wound Care Centres
- Cancer Care Centres
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Cell and Gene Therapy Market Scenario
The market is expected to experience significant growth in the coming years. The market is driven by the increasing prevalence of chronic diseases, advancements in gene editing technologies, and the rising demand for personalized medicine.North America dominates the cell and gene therapy market, followed by Europe and the Asia Pacific. The high prevalence of chronic diseases, coupled with favourable government policies and the availability of advanced healthcare infrastructure, is driving the growth of the market in North America and Europe. The Asia Pacific region is expected to grow at the highest CAGR during the forecast period, owing to the increasing prevalence of chronic diseases and rising healthcare expenditure in the region.
The oncology segment dominates the cell and gene therapy market by therapeutic application, followed by cardiovascular diseases and central nervous system disorders. The increasing prevalence of cancer and the rising demand for innovative cancer treatments are driving the growth of the oncology segment. The cell therapy segment dominates the market by therapeutic modality, followed by gene therapy and tissue engineering. The increasing demand for personalized medicine and the growing number of clinical trials are driving the growth of the cell therapy segment.
Key Players in the Global Cell and Gene Therapy Market
The report gives an in-depth analysis of the key players involved in the cell and gene therapy market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- Amgen, Inc
- Bluebird Bio, Inc
- Castle Creek Pharmaceutical Holdings
- Kite Pharma, Inc
- Novartis AG
- Orchard Therapeutics plc
- Pfizer, Inc
- Spark Therapeutics, Inc
- Vericel Corporation
- Human Stem Cells Institute
- Kolon Tissuegene Inc
- Organogenesis Holdings Inc
Table of Contents
1 Preface
3 Global Cell and Gene Therapy Market Overview
4 Global Cell and Gene Therapy Market Landscape
5 Global Cell and Gene Therapy Market Dynamics
6 Global Cell and Gene Therapy Market Segmentation
7 North America Cell and Gene Therapy Market
8 Europe Cell and Gene Therapy Market
9 Asia Pacific Cell and Gene Therapy Market
10 Latin America Cell and Gene Therapy Market
11 Middle East and Africa Cell and Gene Therapy Market
12 Patent Analysis
13 Grants Analysis
14 Funding Analysis
15 Partnership and Collaborations Analysis
16 Regulatory Framework
17 Supplier Landscape
18 Global Cell and Gene Therapy Market - Distribution Model (Additional Insight)
20 Company Competitiveness Analysis (Additional Insight)
21 Payment Methods (Additional Insight)
Companies Mentioned
- Amgen, Inc.
- Bluebird Bio, Inc.
- Castle Creek Pharmaceutical Holdings
- Kite Pharma, Inc.
- Novartis AG
- Orchard Therapeutics plc.
- Pfizer, Inc.
- Spark Therapeutics, Inc.
- Vericel Corporation
- Vericel Corporation
- Human Stem Cells Institute
- Kolon Tissuegene Inc.
- Organogenesis Holdings Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | April 2023 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 21.45 Billion |
| Forecasted Market Value ( USD | $ 113.65 Billion |
| Compound Annual Growth Rate | 23.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |