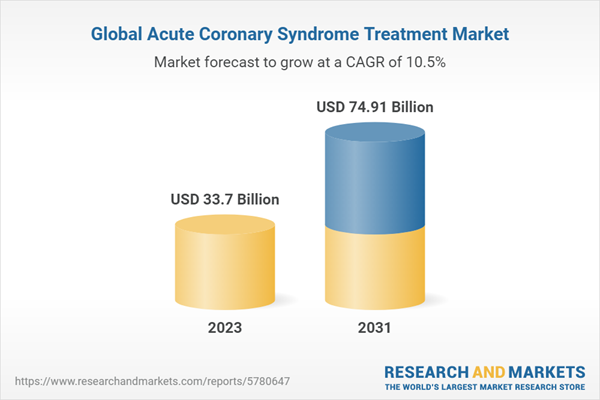
The global acute coronary syndrome treatment market size was valued at USD 30.5 billion in 2022 and is projected to grow at a CAGR of 10.5% during the forecast period of 2023-2031 to reach a value of USD 74.91 billion by 2031. The market growth can be attributed to the increasing prevalence of cardiovascular diseases and the rising geriatric population.
Technological advancements play a significant role in the growth of the acute coronary syndrome treatment market. The development of novel treatment options, such as drug-eluting stents and novel anticoagulant drugs, have improved the outcomes for patients with ACS. Additionally, the increasing adoption of telemedicine and remote monitoring has made it easier for patients to access care and for healthcare providers to monitor their patients' conditions. This has expanded the reach of ACS treatment and helped to meet the growing demand for services.
The acute coronary syndrome treatment market is expected to continue to grow in the coming years, as the global prevalence of cardiovascular diseases continues to rise, and new treatment options and technologies are developed to meet the needs of patients. However, there are still significant barriers to accessing ACS treatment, particularly in low- and middle-income countries, where access to healthcare services is often limited. Efforts are underway to address these barriers and improve access to acute coronary syndrome treatment globally.
North America is currently the largest market for acute coronary syndrome treatment, accounting for a significant share of the global market. The region's large market share is driven by factors such as a high prevalence of cardiovascular diseases, a well-established healthcare infrastructure, and strong investment in research and development. In addition, the growing adoption of digital healthcare technologies, such as telemedicine and remote monitoring, is further driving the growth of the market in North America.
Europe is also a significant market for acute coronary syndrome treatment, the market growth is driven by factors such as a large population base, increasing awareness of cardiovascular diseases, and government initiatives to address these health concerns. Additionally, the region has a well-established healthcare system, which supports the delivery of ACS treatment.
Asia Pacific is another region that is experiencing significant growth in the acute coronary syndrome treatment market. The region's market growth is driven by factors such as a large population base, increasing prevalence of cardiovascular diseases, and the growing adoption of digital healthcare technologies. In addition, government initiatives to address cardiovascular diseases are helping to drive the growth of the market in the region.
Global Acute Coronary Syndrome Treatment Market: Introduction
Acute coronary syndrome (ACS) treatment refers to the medical care provided to individuals experiencing a sudden reduction or blockage of blood flow to the heart. ACS can include conditions such as unstable angina, non-ST elevation myocardial infarction (NSTEMI), and ST-elevation myocardial infarction (STEMI). The increasing prevalence of cardiovascular diseases, such as coronary artery disease, hypertension, and obesity, is driving the demand for ACS treatment. Other factors such as the rising geriatric population, advancements in treatment options, and increasing government initiatives are also expected to drive market growth.Technological advancements play a significant role in the growth of the acute coronary syndrome treatment market. The development of novel treatment options, such as drug-eluting stents and novel anticoagulant drugs, have improved the outcomes for patients with ACS. Additionally, the increasing adoption of telemedicine and remote monitoring has made it easier for patients to access care and for healthcare providers to monitor their patients' conditions. This has expanded the reach of ACS treatment and helped to meet the growing demand for services.
The acute coronary syndrome treatment market is expected to continue to grow in the coming years, as the global prevalence of cardiovascular diseases continues to rise, and new treatment options and technologies are developed to meet the needs of patients. However, there are still significant barriers to accessing ACS treatment, particularly in low- and middle-income countries, where access to healthcare services is often limited. Efforts are underway to address these barriers and improve access to acute coronary syndrome treatment globally.
Acute Coronary Syndrome Epidemiology
According to the World Health Organization, cardiovascular diseases are the leading cause of death globally, accounting for an estimated 17.9 million deaths each year. Acute coronary syndrome is a significant contributor to this mortality, with millions of people experiencing ACS events annually. The incidence of ACS is expected to continue to rise, driven by factors such as aging populations, increasing prevalence of risk factors such as obesity, and lifestyle changes.Acute Coronary Syndrome Treatment Market Segmentations
The market can be segmented based on indication, class, treatment channel, and region:Market Breakup by Indication
- Vial Unstable Angina
- ST-Elevation Myocardial Infarction
- Non-ST-Elevation Myocardial Infarction
- Others
Market Breakup by Class
- Anti-Hypertensive
- Antithrombotic
- Statins
- Others
Market Breakup by Treatment Channel
- Public
- Private
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Acute Coronary Syndrome Treatment Market Analysis
The global acute coronary syndrome treatment market has experienced significant growth over the past few years, driven by factors such as the increasing prevalence of cardiovascular diseases, rising geriatric population, and advancements in treatment options. One key driver of growth in the ACS treatment market is the increasing prevalence of cardiovascular diseases.North America is currently the largest market for acute coronary syndrome treatment, accounting for a significant share of the global market. The region's large market share is driven by factors such as a high prevalence of cardiovascular diseases, a well-established healthcare infrastructure, and strong investment in research and development. In addition, the growing adoption of digital healthcare technologies, such as telemedicine and remote monitoring, is further driving the growth of the market in North America.
Europe is also a significant market for acute coronary syndrome treatment, the market growth is driven by factors such as a large population base, increasing awareness of cardiovascular diseases, and government initiatives to address these health concerns. Additionally, the region has a well-established healthcare system, which supports the delivery of ACS treatment.
Asia Pacific is another region that is experiencing significant growth in the acute coronary syndrome treatment market. The region's market growth is driven by factors such as a large population base, increasing prevalence of cardiovascular diseases, and the growing adoption of digital healthcare technologies. In addition, government initiatives to address cardiovascular diseases are helping to drive the growth of the market in the region.
Key Players in the Global Acute Coronary Syndrome Treatment Market
The report provides a detailed analysis of the key players involved in the acute coronary syndrome treatment market, including their business overview, product portfolio, recent developments, and financial analysis. Some of the major players operating in the market include:- Pfizer Inc
- Sanofi S.A
- GSK plc
- Merck & Co., Inc
- AbbVie Inc
- Amgen Inc
- Arena Pharmaceuticals
- AstraZeneca plc
- Baxter International Inc
- Bayer AG
- Biogen Inc
- C. H. Boehringer Sohn Co. KG
- Eli Lilly and Company
- Gilead Sciences, Inc
- Teva Pharmaceutical Industries Ltd
Table of Contents
1 Preface
4 Global Acute Coronary Syndrome Overview
5 Patient Profile
6 Global Acute Coronary Syndrome Treatment Market
7 Current Scenario Evaluation and Regulatory Framework
8 Challenges & Unmet Needs
9 Global Acute Coronary Syndrome Treatment Market Dynamics
10 Supplier Landscape
12 Global Acute Coronary Syndrome Treatment Drugs Distribution Model (Additional Insight)
13 Payment Methods (Additional Insight)
Companies Mentioned
- Pfizer Inc.
- Sanofi S.A.
- GSK plc
- Merck & Co., Inc.
- Amgen Inc.
- Arena Pharmaceuticals
- AstraZeneca plc
- Baxter International Inc.
- Bayer AG
- Biogen Inc.
- C. H. Boehringer Sohn Co. KG
- Eli Lilly and Company
- Gilead Sciences, Inc.
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 147 |
| Published | April 2023 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 33.7 Billion |
| Forecasted Market Value ( USD | $ 74.91 Billion |
| Compound Annual Growth Rate | 10.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |