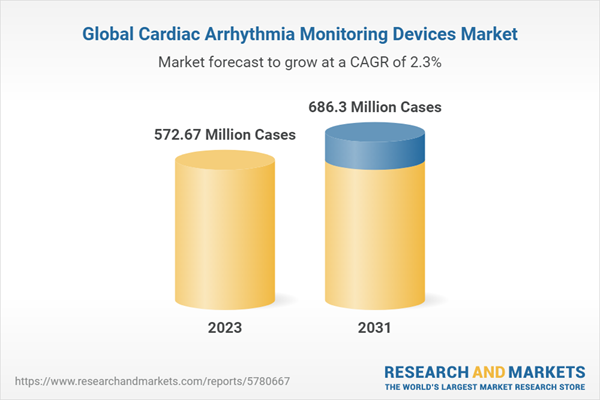
The rising prevalence of cardiovascular diseases has been value of the cardiac arrhythmia monitoring devices market. The number of cardiovascular-diagnosed cases was nearly 559.8 million in 2022. The prevalence of the disease is likely to grow at a rate of 2.3% in the forecast period of 2023-2031. With this growth rate, the number of cases is likely to reach close to 686.3 million by 2031.
The electrocardiogram (ECG) monitors segment is expected to hold the largest market share during the forecast period, owing to its widespread adoption in healthcare settings. The homecare settings segment is expected to witness the highest growth rate, driven by the growing trend of remote patient monitoring and the increasing demand for portable and wearable monitoring devices.
North America dominates the cardiac arrhythmia monitoring devices market, followed by Europe and the Asia Pacific region. The high prevalence of cardiovascular diseases, the availability of advanced healthcare infrastructure, and the presence of key market players are driving the growth of the market in these regions. The Asia Pacific region is expected to witness the highest growth rate during the forecast period, driven by the increasing geriatric population and the growing demand for advanced monitoring devices.
The COVID-19 pandemic has also affected the cardiac arrhythmia monitoring devices market, with disruptions in supply chains and manufacturing activities. However, the market is expected to recover in the post-pandemic period, driven by the growing need for effective cardiovascular disease management.
Cardiac Arrhythmia Monitoring Devices Market: Introduction
Cardiac arrhythmia is a condition that occurs when the heart beats too fast, too slow, or irregularly. It is a common cardiovascular disease that affects individuals of all age groups, and its prevalence has been increasing in recent years. The market for cardiac arrhythmia monitoring devices includes a range of products such as electrocardiogram (ECG) monitors, Holter monitors, event monitors, and implantable cardiac monitors. The growing demand for advanced monitoring devices and the increasing prevalence of cardiovascular diseases are driving the growth of the market.cardiac Arrhythmia Monitoring Devices Market Segmentations
The market can be categorized into type, application, distribution channel, and region.Market Breakup by Type
- ECG Monitors
- Holter Monitors
- Implantable Monitors
- Mobile Cardiac Telemetry
- Others
Market Breakup by Application
- Atrial Fibrillation
- Bradycardia
- Premature Contraction
- Tachycardia
- Ventricular Fibrillation
- Others
Market Breakup by Distribution Channels
- Hospitals and Clinics
- Ambulatory Surgery Centres
- Diagnostic Centres
- Homecare Settings
Cardiac Arrhythmia Monitoring Devices Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Cardiac Arrhythmia Monitoring Devices Market Scenario
The increasing prevalence of cardiovascular diseases, technological advancements in monitoring devices, and the growing geriatric population are expected to drive the growth of the market.The electrocardiogram (ECG) monitors segment is expected to hold the largest market share during the forecast period, owing to its widespread adoption in healthcare settings. The homecare settings segment is expected to witness the highest growth rate, driven by the growing trend of remote patient monitoring and the increasing demand for portable and wearable monitoring devices.
North America dominates the cardiac arrhythmia monitoring devices market, followed by Europe and the Asia Pacific region. The high prevalence of cardiovascular diseases, the availability of advanced healthcare infrastructure, and the presence of key market players are driving the growth of the market in these regions. The Asia Pacific region is expected to witness the highest growth rate during the forecast period, driven by the increasing geriatric population and the growing demand for advanced monitoring devices.
The COVID-19 pandemic has also affected the cardiac arrhythmia monitoring devices market, with disruptions in supply chains and manufacturing activities. However, the market is expected to recover in the post-pandemic period, driven by the growing need for effective cardiovascular disease management.
Key Players in the Global Cardiac Arrhythmia Monitoring Devices Market
The report gives an in-depth analysis of the key players involved in the cardiac Arrhythmia monitoring devices market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- AliveCor, Inc
- BioTelemetry, Inc
- Fukuda Denshi Co., Ltd
- GE Healthcare
- BIOTRONIK SE & Co KG
- Biotricity
- iRhythm Technologies, Inc
- Medtronic plc
- Spacelabs Healthcare
- Welch Allyn, Inc. (Hillrom Services, Inc)
Table of Contents
1 Preface
4 Global Cardiac Arrhythmia Monitoring Devices Market
5 Trade Data Analysis by HS Code
6 Current Scenario Evaluation and Regulatory Framework
7 Global Cardiac Arrhythmia Monitoring Devices Market Dynamics
8 Supplier Landscape
10 Pricing Models and Strategies (Additional Insight)
11 Global Cardiac Arrhythmia Monitoring Devices Distribution Model (Additional Insight)
Companies Mentioned
- AliveCor, Inc.
- BioTelemetry, Inc.
- Fukuda Denshi Co., Ltd.
- GE Healthcare
- BIOTRONIK SE & Co. KG
- Biotricity
- iRhythm Technologies, Inc.
- Medtronic plc
- Spacelabs Healthcare
- Welch Allyn, Inc. (Hillrom Services, Inc.)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | April 2023 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value in 2023 | 572.67 Million Cases |
| Forecasted Market Value by 2031 | 686.3 Million Cases |
| Compound Annual Growth Rate | 2.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |