Hepatitis B Infection Treatment Market Overview
Hepatitis B infection treatment focuses on suppressing the virus and preventing liver damage. According to the World Health Organization’s 2024 Global Hepatitis Report, hepatitis B diagnosis rates increased from 10% in 2019 to 13% in 2024, while treatment coverage rose from just 2% to 3% globally. Regionally, the WHO African Region bears the highest burden, accounting for 63% of new hepatitis B infections. However, only 18% of newborns receive the birth-dose vaccination, a critical intervention to prevent early transmission. In the Western Pacific Region (which accounts for 47% of global hepatitis B deaths) treatment coverage is only 23% among those diagnosed, far below levels needed to significantly reduce mortality. Current options include antiviral drugs like tenofovir and entecavir, immunomodulators, and supportive therapies. While no complete cure exists, long-term treatment can manage chronic cases effectively. Vaccination plays a vital role in prevention. Advancements in drug development, rising awareness, and government initiatives are expanding access to care.Hepatitis B Infection Treatment Market Growth Drivers
Adoption of Gene-Editing Therapies to Boost Market Growth
Increasing demand for curative therapies and growing investment in gene-editing technologies are driving innovation in the landscape. For instance, in March 2025, Precision BioSciences announced that the U.S. FDA cleared its Investigational New Drug (IND) application for PBGENE-HBV, a meganuclease-edited in vivo therapeutic developed using its proprietary ARCUS® platform. The clearance enables expansion of the ELIMINATE-B trial into the U.S., marking the first investigational in vivo gene-editing therapy approved to enter a clinical trial for chronic hepatitis B in the country. This breakthrough positions gene-editing-based therapies as a promising frontier in the forecast period, likely shifting the market toward potentially curative treatments and attracting significant clinical and commercial interest.Hepatitis B Infection Treatment Market Trends
Some of the notable trends include the use of combination therapies for enhanced efficacy of treatment, rise in clinical trials, focus on preventing the condition with prompt vaccination.Surge in Clinical Trial Activities to Drive Market Growth to Meet Rising Hepatitis B Treatment Market Demand
In May 2025, Vir Biotechnology announced promising results from its MARCH Phase 2 trial. The trial evaluated tobevibart and elebsiran, both with and without PEG-IFNα, in patients with baseline HBsAg < 1,000 IU/mL. Around 17%-21% of patients achieved undetectable HBsAg at 24 weeks post-treatment. These results highlight growing efficacy of combination approaches and support continued investment in immunotherapeutic strategies, which may redefine treatment expectations and improve functional cure rates in the coming years.
Emphasis on Vaccine-Driven Prevention to Boost Hepatitis B Treatment Market Value
In April 2024, the WHO reported that hepatitis B infected 254 million people globally in 2022, with 1.1 million deaths annually. The disease remains highly transmissible through childbirth and unsafe medical practices. However, widespread use of highly effective vaccines is significantly reducing new infections. As national immunization programs expand, especially in high-burden regions, prevention strategies are likely to influence future treatment demand, reshaping the hepatitis B market’s trajectory and supporting sustained global health outcomes.Use of Combination Therapies to Accelerate Hepatitis B Treatment Market Growth
In October 2021, Assembly Biosciences and Antios Therapeutics launched a clinical collaboration to study a triple-combination therapy involving tenofovir disoproxil fumarate, ATI-2173, and AB-729 for chronic hepatitis B. The strategic use of nucleotide analogs, polymerase inhibitors, and capsid assembly modulators is expected to drive higher functional cure rates. The increasing success of multi-drug regimens will likely influence future standard-of-care models and attract broader investment into combination-based therapeutics, thus boosting market growth.Hepatitis B Infection Treatment Market Segmentation
Hepatitis B Infection Treatment Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- Acute
- Chronic
Market Breakup by Treatment Type
- Immunomodulator Drugs
- Vaccines
- Antiviral Drugs
- Surgery
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Hepatitis B Infection Treatment Market Share
Chronic Type to Dominate the Share by Type
Chronic hepatitis B infection is poised to hold the largest market share by type due to its long-term nature and global prevalence, especially in regions like Asia and sub-Saharan Africa. The increasing burden of chronic cases requires ongoing treatment and monitoring, fuelling consistent demand for therapeutic drugs and diagnostics. With growing awareness and improved diagnosis rates, this segment is expected to drive market expansion through the forecast period, supported by government-led screening and vaccination programs.Antiviral Drugs to Hold a Substantial Hepatitis B Infection Treatment Market Value for Segmentation by Treatment Type
Antiviral drugs will likely dominate the treatment type segment, driven by their effectiveness in suppressing hepatitis B virus replication and preventing disease progression. As per the analysis by Expert Market Research, the global antiviral drugs market is anticipated to grow at a CAGR of 4.10% during the forecast period of 2025-2034. These drugs, especially tenofovir and entecavir, are widely prescribed due to their safety profiles and long-term efficacy. With rising treatment accessibility and strong pipeline development, this segment will continue to grow. Increased funding for drug development and broader adoption in high-burden regions are key factors sustaining its leadership in the global market.
Oral Route to Dominate the Share by Route of Administration
The oral route is expected to hold the largest market share by route of administration, primarily due to patient preference, ease of use, and better compliance. Most antiviral hepatitis B treatments are available in oral formulations, which facilitate long-term therapy for chronic cases. The convenience of self-administration at home reduces hospital dependency, supporting broader access. Future market growth will be reinforced by the development of novel oral formulations with improved bioavailability and safety profiles.Hepatitis B Infection Treatment Market Analysis by Region
The United States is expected to lead the market, driven by high awareness, advanced diagnostics, and strong pharmaceutical presence. Major players like Gilead and GlaxoSmithKline dominate with antivirals such as tenofovir and entecavir. Widespread screening programs, immigration from endemic regions, and robust insurance coverage support market growth. In Europe, Germany has a structured healthcare system and offers full reimbursement for hepatitis B therapies. The country emphasizes early diagnosis and consistent long-term management using antivirals. Public awareness and screening efforts are strong, and the presence of multinational pharmaceutical companies ensures access to the latest treatments.Leading Players in the Hepatitis B Infection Treatment Market
The key features of the market report comprise clinical trials and pipeline analysis, patent analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Accord Healthcare (Intas Pharmaceuticals Ltd)
Headquartered in the United Kingdom and operating as a subsidiary of Intas Pharmaceuticals (founded in 1984 in India), Accord Healthcare offers a wide range of affordable generic medicines, including treatments for hepatitis B. The company is known for its strong presence in oncology, cardiology, and infectious diseases. Its extensive manufacturing and R&D capabilities enable the timely development of antiviral drugs. Accord’s global distribution and compliance with international regulatory standards strengthen its role in hepatitis B infection management.Apotex Inc. (SK Capital Partners)
Founded in 1974 and based in Toronto, Canada, Apotex Inc. is a prominent pharmaceutical company now under the ownership of SK Capital Partners. It is known for producing high-quality generic medications across various therapeutic areas, including hepatitis B. Apotex offers cost-effective antiviral drugs and actively supplies healthcare systems globally. Its research-driven approach, coupled with consistent regulatory approvals, supports the development of reliable treatment options in the hepatitis B infection treatment landscape.Aurobindo Pharma
Established in 1986 and headquartered in Hyderabad, India, Aurobindo Pharma is a leading global pharmaceutical manufacturer with a strong presence in antiviral and infectious disease segments. The company’s hepatitis B portfolio includes key antiviral medications like tenofovir and lamivudine. Aurobindo’s vertically integrated business model, global regulatory compliance, and robust R&D infrastructure position it as a vital player in the hepatitis B treatment market, catering to both developed and emerging regions.Bristol-Myers Squibb Company
Founded in 1887 and headquartered in New York, USA, Bristol-Myers Squibb is a major biopharmaceutical company known for pioneering innovation in immunology, oncology, and virology. In the hepatitis B market, it is best known for Baraclude (entecavir), a leading antiviral therapy. BMS invests heavily in clinical research and strategic collaborations to advance its treatment offerings. With a focus on precision medicine and patient-centered care, the company remains a key contributor to hepatitis B treatment globally.Other key players in the market include Gilead Sciences, Inc., GSK plc, Lupin, Merck & Co., Inc., Teva Pharmaceutical Industries Ltd, and Zydus Healthcare Limited.
Key Questions Answered in the Hepatitis B Infection Treatment Market
- What was the hepatitis B infection treatment market value in 2024?
- What is the hepatitis B infection treatment market forecast outlook for 2025-2034?
- What are the major factors aiding the hepatitis B infection treatment demand?
- How has the market performed so far, and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major hepatitis B infection treatment market trends?
- Which type will lead the market segment?
- Which treatment type will lead the market segment?
- Which route of administration will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the hepatitis B infection treatment market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Accord Healthcare (Intas Pharmaceuticals Ltd.)
- Apotex Inc. (SK Capital Partners)
- Aurobindo Pharma
- Bristol-Myers Squibb Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
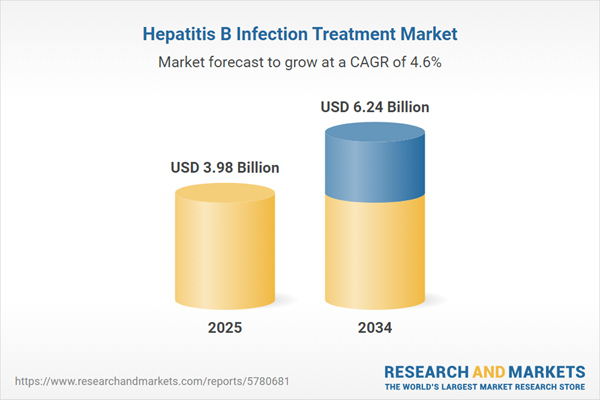
| Estimated Market Value ( USD | $ 3.98 Billion |
| Forecasted Market Value ( USD | $ 6.24 Billion |
| Compound Annual Growth Rate | 4.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |