Point of Care Coagulation Testing Devices: Introduction
Point of Care Coagulation Testing Devices are handheld or portable devices used to measure blood clotting parameters such as prothrombin time (PT), activated partial thromboplastin time (aPTT), and international normalized ratio (INR) at the patient's bedside. These devices are used to monitor patients who are at risk of bleeding or thrombosis and require anticoagulant therapy.Point of Care Coagulation Testing Devices Market Scenario
The global Point of Care Coagulation Testing Devices market is growing due to an increase in the incidence of blood clotting disorders such as deep vein thrombosis, pulmonary embolism, and stroke. The increasing demand for early diagnosis and treatment of these disorders is driving market growth. Additionally, the rising geriatric population and increasing surgical procedures are expected to further fuel market growth.The market is also witnessing an increasing trend towards the development of technologically advanced devices that provide accurate and rapid results. Manufacturers are focusing on developing devices that are easy to use and require minimal training. Furthermore, the development of wireless and mobile devices is expected to create new growth opportunities in the market.
Overall, the global Point of Care Coagulation Testing Devices market is expected to continue its growth trajectory due to increasing demand for early diagnosis and treatment of blood clotting disorders and technological advancements in the devices.
Point of Care Coagulation Testing Devices Market Segmentations
Market Breakup by Device Type
- Anticoagulation Monitoring Devices
- Platelet Function Monitoring Devices
Viscoelastic Coagulation Monitoring Devices
- Thromboelastography (TEG)
- Rotational Thromboelastometry (ROTEM)
- Others
Market Breakup by End User
- Hospitals & Clinics
- Homecare
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Key Trends in the Point of Care Coagulation Testing Devices Market
The market for point-of-care coagulation testing devices is growing steadily due to the increasing prevalence of coagulation disorders and the rising demand for rapid diagnostic tools. Some of the key trends in the market include:- Technological advancements: There has been a significant focus on developing advanced point-of-care coagulation testing devices that offer accurate and reliable results in a shorter amount of time. Manufacturers are also integrating wireless and cloud-based connectivity features to enable remote monitoring and data analysis
- Increasing demand for home-based testing: With the growing trend of self-monitoring and the shift towards home-based care, there is a rising demand for portable and easy-to-use coagulation testing devices that patients can use at home
- Growing adoption of anticoagulant therapies: The increasing adoption of anticoagulant therapies for the prevention and treatment of thrombosis is expected to drive the demand for point-of-care coagulation testing devices. These devices help in monitoring the effects of these therapies and adjusting the dosage accordingly
- Rising geriatric population: The aging population is more prone to coagulation disorders, and as the geriatric population continues to grow, the demand for point-of-care coagulation testing devices is expected to increase
- Increasing prevalence of cardiovascular diseases: The growing prevalence of cardiovascular diseases, which often require coagulation testing, is expected to drive the demand for point-of-care coagulation testing devices
Point of Care Coagulation Testing Devices Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Teleflex Incorporated
- F.Hoffmann-La Roche AG
- Abbott Laboratories
- Siemens Healthineers
- Medtronic
- Haemonetics Corporation
- Hemosonics, LLC
- Micropoint Bioscieces, Inc
- Werfen
- Sienco, Inc
- Koninklijke Philips N.V.
Table of Contents
Companies Mentioned
- Teleflex Incorporated
- F.Hoffmann-La Roche AG
- Abbott Laboratories
- Siemens Healthineers
- Medtronic
- Haemonetics Corporation
- Hemosonics, LLC
- Micropoint Bioscieces, Inc.
- Werfen
- Sienco, Inc.
- Koninklijke Philips N.V.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2023 |
| Forecast Period | 2023 - 2031 |
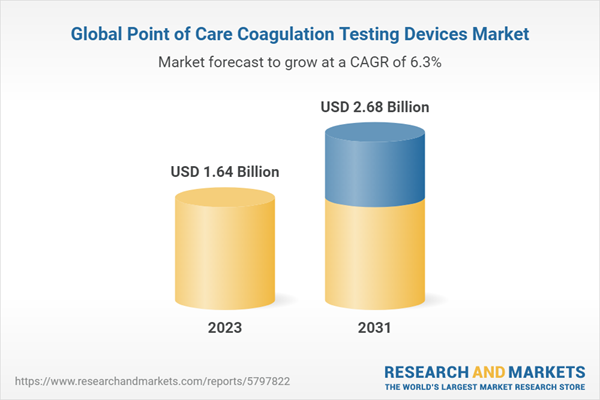
| Estimated Market Value ( USD | $ 1.64 Billion |
| Forecasted Market Value ( USD | $ 2.68 Billion |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |