Conjugate Vaccine Market: Introduction
A conjugate vaccine is a type of vaccine that is created by linking a protein or polysaccharide from a disease-causing bacterium or virus to a carrier molecule. This process creates a new molecule that the body's immune system recognizes as foreign and mounts an immune response against it. Conjugate vaccines are used to prevent bacterial infections, particularly in children, and are known to be highly effective.The primary use of conjugate vaccines is to prevent bacterial infections caused by bacteria that have a polysaccharide capsule. These bacteria are difficult for the immune system to recognize and attack because they are coated with a polysaccharide layer that the immune system does not recognize as foreign. However, when the polysaccharide is linked to a carrier protein, the immune system recognizes it as a foreign invader and produces an immune response against it.
Some examples of bacterial infections that can be prevented with conjugate vaccines include meningitis, pneumonia, and sepsis caused by Streptococcus pneumoniae, Haemophilus influenzae type b (Hib), and Neisseria meningitidis. Conjugate vaccines are also used to prevent infections caused by the bacteria that cause diphtheria and tetanus.
Benefits of conjugate vaccines include:
- High efficacy: Conjugate vaccines are highly effective at preventing bacterial infections and have been shown to be more effective than other types of vaccines
- Long-lasting protection: Conjugate vaccines provide long-lasting protection against bacterial infections, reducing the need for additional doses or boosters
- Fewer side effects: Conjugate vaccines are known to have fewer side effects than other types of vaccines, making them a safe and effective option for immunization
- Reduced healthcare costs: Conjugate vaccines can help reduce healthcare costs by preventing bacterial infections that require expensive medical treatment, hospitalization, and rehabilitation
conjugate Vaccine Market Segmentations
The market can be categorised into type, indications, pathogen type, patient type, end user, and region.Market Breakup by Type
- Monovalent Conjugate Vaccines
- Multivalent Conjugate Vaccines
Market Breakup by Indications
- Pneumococcal Disease
- Influenza
- Meningococcal Disease
- Diphtheria Tetanus and Pertussis (DTP)
- Others
Market Breakup by Pathogen Type
- Bacterial Conjugate Vaccine
- Viral Conjugate Vaccine
- Combination (Viral and Bacterial)
Market Breakup by Patient type
- Pediatrics
- Adults
Market Breakup by End User
- Hospitals
- Clinics
- Homecare Settings
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Conjugate Vaccine Market Scenario
The global market for conjugate vaccine is a growing segment of the pharmaceutical industry, driven by increasing demand for effective vaccines to prevent bacterial infections. Conjugate vaccines are used to prevent bacterial infections caused by bacteria that have a polysaccharide capsule. They are particularly effective in preventing infections in children, and are used to prevent diseases such as meningitis, pneumonia, and sepsis caused by Streptococcus pneumoniae, Haemophilus influenzae type b (Hib), and Neisseria meningitidis.The market for conjugate vaccines is expected to continue to grow at a steady pace, driven by several factors, including increasing awareness about the importance of vaccination, the development of new and innovative vaccines, and the increasing adoption of vaccines in emerging markets.
North America is the largest market for conjugate vaccines, owing to the high prevalence of bacterial infections and well-established healthcare infrastructure in the region. However, the Asia Pacific region is expected to witness significant growth in the coming years, driven by the increasing focus on healthcare development and rising demand for cost-effective medical devices. Countries like China and India are expected to be major contributors to this growth due to their large populations and increasing healthcare spending.
The market for conjugate vaccines is highly competitive, with many players offering a wide range of products to cater to the diverse needs of healthcare providers and patients. Some of the key players in the market include Pfizer, Merck & Co., Inc., GlaxoSmithKline plc, Sanofi Pasteur, and Novartis International AG.
Overall, the conjugate vaccine market is expected to continue to grow at a steady pace, driven by the increasing demand for safe and effective healthcare delivery. With increasing awareness about the importance of vaccination and the development of new and innovative vaccines, the adoption of conjugate vaccines is likely to increase in the coming years, further fuelling the growth of the market.
Key Players in the Global Conjugate Vaccine Market
The report gives an in-depth analysis of the key players involved in the conjugate vaccine market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- Merck & Co., Inc
- Novartis AG
- Serum Institute of India Pvt. Ltd
- Pfizer Inc
- Sanofi
- Bharat Biotech
- Biological E
- GlaxoSmithKline plc
- Astellas Pharma Inc
- AstraZeneca
- SutroVax Inc
- CSL Limited
- Bavarian Nordic
- Emergent BioSolutions Inc
- Valneva SE
- Moderna, Inc
- Novavax
- Johnson & Johnson Services, Inc
- Themis Bioscience GmbH
- B. Braun Melsungen AG
Table of Contents
Companies Mentioned
- Merck & Co. Inc.
- Novartis AG
- Serum Institute of India Pvt. Ltd.
- Pfizer Inc.
- Sanofi
- Bharat Biotech
- Biological E
- GlaxoSmithKline plc.
- Astellas Pharma Inc.
- Astrazeneca
- Sutrovax Inc.
- Csl Limited
- Bavarian Nordic
- Emergent Biosolutions Inc.
- Valneva Se
- Moderna, Inc.
- Novavax
- Johnson & Johnson Services, Inc.
- Themis Bioscience GmbH
- B. Braun Melsungen Ag
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 147 |
| Published | April 2023 |
| Forecast Period | 2023 - 2031 |
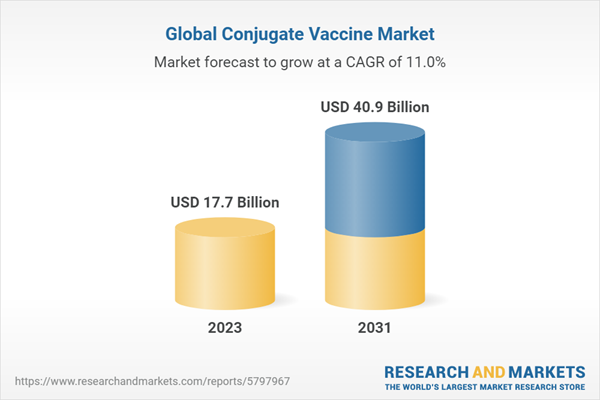
| Estimated Market Value ( USD | $ 17.7 Billion |
| Forecasted Market Value ( USD | $ 40.9 Billion |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |